
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum.

Cysteine dioxygenase (CDO) is a non-heme iron enzyme that catalyzes the conversion of L-cysteine to cysteine sulfinic acid. CDO plays an important role in cysteine catabolism, regulating intracellular levels of cysteine and responding changes in cysteine availability. As such, CDO is highly regulated and undergoes large changes in concentration and efficiency. It oxidizes cysteine to the corresponding sulfinic acid by activation of dioxygen, although the exact mechanism of the reaction is still unclear. In addition to being found in mammals, CDO also exists in some yeast and bacteria, although the exact function is still unknown. CDO has been implicated in various neurodegenerative diseases and cancers, which is likely related to cysteine toxicity.
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14.

Catechol 1,2- dioxygenase is an enzyme that catalyzes the oxidative ring cleavage of catechol to form cis,cis-muconic acid:
In enzymology, a biphenyl 2,3-dioxygenase (EC 1.14.12.18) is an enzyme that catalyzes the chemical reaction
In enzymology, a naphthalene 1,2-dioxygenase (EC 1.14.12.12) is an enzyme that catalyzes the chemical reaction

Nitric oxide dioxygenase (EC 1.14.12.17) is an enzyme that catalyzes the conversion of nitric oxide (NO) to nitrate (NO−
3) . The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:
In enzymology, a taurine dioxygenase (EC 1.14.11.17) is an enzyme that catalyzes the chemical reaction.
In enzymology, a 3-hydroxy-2-methylquinolin-4-one 2,4-dioxygenase (EC 1.13.11.48) is an enzyme that catalyzes the chemical reaction
Acireductone dioxygenase [iron(II)-requiring] (EC 1.13.11.54) is an enzyme that catalyzes the chemical reaction
Acireductone dioxygenase (Ni2+-requiring) (EC 1.13.11.53) is an enzyme that catalyzes the chemical reaction
In enzymology, tryptophan 2,3-dioxygenase (EC 1.13.11.11) is a heme enzyme that catalyzes the oxidation of L-tryptophan (L-Trp) to N-formyl-L-kynurenine, as the first and rate-limiting step of the kynurenine pathway.
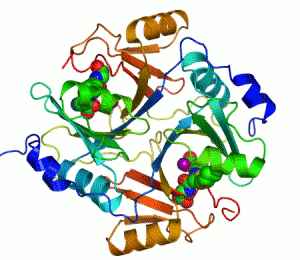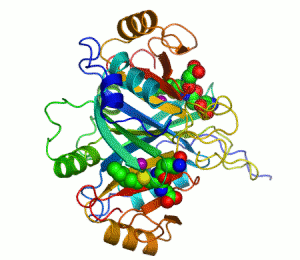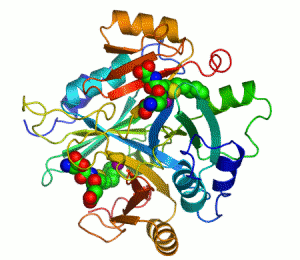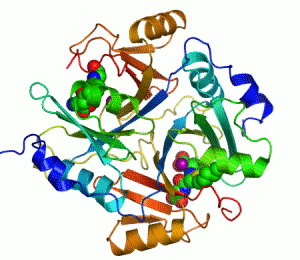
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal.
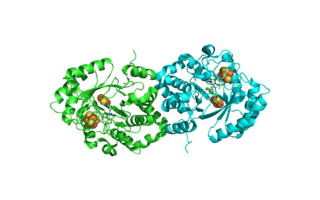
Biotin synthase (BioB) is an enzyme that catalyzes the conversion of dethiobiotin (DTB) to biotin; this is the final step in the biotin biosynthetic pathway. Biotin, also known as vitamin B7, is a cofactor used in carboxylation, decarboxylation, and transcarboxylation reactions in many organisms including humans. Biotin synthase is an S-Adenosylmethionine (SAM) dependent enzyme that employs a radical mechanism to thiolate dethiobiotin, thus converting it to biotin.

Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine triphosphate (ATP) generation to xenobiotic degradation, the use of dioxygen as a biological oxidant is widespread and varied in the exact mechanism of its use. Enzymes employ many different schemes to use dioxygen, and this largely depends on the substrate and reaction at hand.
D-xylose reductase (EC 1.1.1.307, XylR, XyrA, msXR, dsXR, monospecific xylose reductase, dual specific xylose reductase, NAD(P)H-dependent xylose reductase, xylose reductase) is an enzyme with systematic name xylitol:NAD(P)+ oxidoreductase. This enzyme catalyses the following chemical reaction
Phosphonoacetaldehyde reductase (NADH) (EC 1.1.1.309, PhpC) is an enzyme with systematic name 2-hydroxyethylphosphonate:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction
Methylphosphonate synthase (EC 1.13.11.73, mpnS (gene)) is an enzyme with systematic name 2-hydroxyethylphosphonate:O2 1,2-oxidoreductase (methylphosphonate forming). This enzyme catalyses the following chemical reaction
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.
Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Functionally, the αKG-dependent hydroxylases are comparable to cytochrome P450 enzymes. Both use O2 and reducing equivalents as cosubstrates and both generate water.








