In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.
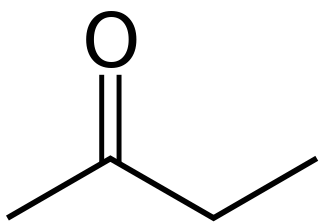
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.

Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.
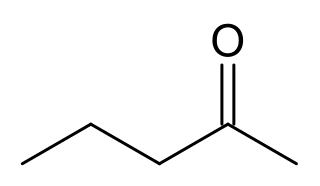
2-Pentanone or methyl propyl ketone (MPK) is a ketone and solvent of minor importance. It is comparable to methyl ethyl ketone, but has a lower solvency and is more expensive. It occurs naturally in Nicotiana tabacum (Tobacco) and blue cheese as a metabolic product of Penicillium mold growth.

3-Pentanone is a simple, symmetrical dialkyl ketone. It is a colorless liquid ketone with an odor like that of acetone. It is soluble in about 25 parts water, but miscible with organic solvents.
Pentanone may refer to the following ketones containing five carbon atoms:
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.

Methyl isobutyl ketone (MIBK) is the organic compound with the formula (CH3)2CHCH2C(O)CH3. This colourless liquid, a ketone, is used as a solvent for gums, resins, paints, varnishes, lacquers, and nitrocellulose.

Mesityl oxide is a α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.

Ethyl isopropyl ketone (2-methyl-3-pentanone) is an aliphatic ketone with used as a reagent in organic chemistry and as a solvent.
The Corey–Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim.
The molecular formula C5H10O may refer to:
The molecular formula C6H12O may refer to:
Heptanone may refer to the following ketones with seven carbon atoms the formula C7H14O:
Hexanone may refer to the following ketones containing six carbon atoms:
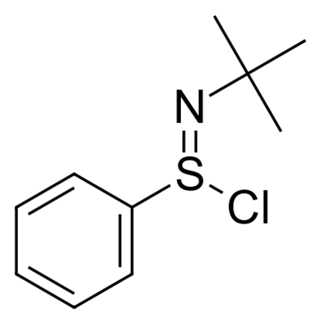
N-tert-Butylbenzenesulfinimidoyl chloride is a useful oxidant for organic synthesis reactions. It is a good electrophile, and the sulfimide S=N bond can be attacked by nucleophiles, such as alkoxides, enolates, and amide ions. The nitrogen atom in the resulting intermediate is basic, and can abstract an α-hydrogen to create a new double bond.

3-Hexanone (ethyl propyl ketone) is an organic compound with the formula C6H12O. It is a ketone used as a solvent and as a chemical intermediate.

3-Methyl-2-pentanone is an aliphatic ketone and isomer of 2-hexanone. It is used as a solvent and as an intermediate for syntheses. Its industrial importance is low. It is produced by base-catalyzed aldol condensation of 2-butanone with acetaldehyde, forming 4-hydroxy-3-methyl-2-pentanone, which is dehydrated to 3-methyl-3-penten-2-one over an acid catalyst, followed by hydrogenation over a palladium catalyst.

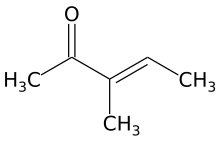
α,β-Unsaturated carbonyl compounds refers to organic compounds with the general structure (O=CR)−Cα=Cβ-R. Examples would be enones and enals. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.