In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
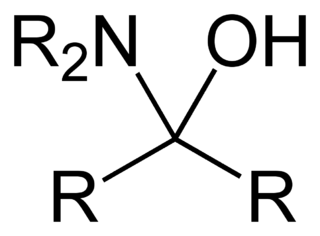
In organic chemistry, a hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: −C(OH)(NR2)−. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution. Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols.

DABCO (1,4-diazabicyclo[2.2.2]octane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis.

N,N′-Dicyclohexylcarbodiimide (DCC or DCCD) is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers upon standing at room temperature, though this mostly occurs with low melting point carbodiimides that are liquids at room temperature. Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis in the presence of water over time.
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH) and NOBF4. The ClO−4 and BF−4 salts are slightly soluble in acetonitrile CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.

Triacetonamine is an organic compound with the formula OC(CH2CMe2)2NH (where Me = CH3). It is a colorless or white solid that melts near room temperature. The compound is an intermediate in the preparation of 2,2,6,6-tetramethylpiperidine, a sterically hindered base and precursor to the reagent called TEMPO. Triacetonamine is formed by the poly-aldol condensation of acetone in the presence of ammonia and calcium chloride:

Sodium methylsulfinylmethylide is the sodium salt of the conjugate base of dimethyl sulfoxide. This unusual salt has some uses in organic chemistry as a base and nucleophile.
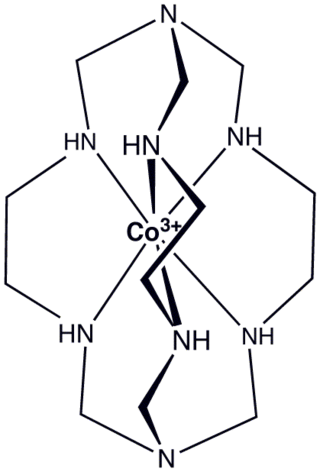
In coordination chemistry, clathrochelates are ligands that encapsulate metal ions. Chelating ligands bind to metals more strongly than related monodentate ligands, and macrocyclic ligands bind more strongly than typical chelating ligands. It follows that bi- or polymacrocyclic ligands would bind to metals particularly strongly. Clathrochelates are usually derived from bimacrocyclic ligands.

1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.
Aminoacetonitrile is the organic compound with the formula NCCH2NH2. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammonium derivative, i.e., [NCCH2NH3]+Cl− and [NCCH2NH3]+HSO4−.

(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl, commonly known as TEMPO, is a chemical compound with the formula (CH2)3(CMe2)2NO. This heterocyclic compound is a red-orange, sublimable solid. As a stable aminoxyl radical, it has applications in chemistry and biochemistry. TEMPO is used as a radical marker, as a structural probe for biological systems in conjunction with electron spin resonance spectroscopy, as a reagent in organic synthesis, and as a mediator in controlled radical polymerization.

Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis.

Glycinamide is a organic compound with the molecular formula H2NCH2C(O)NH2. It is the amide derivative of the amino acid glycine. It is a water-soluble, white solid. Amino acid amides, such as glycinamide are prepared by treating the amino acid ester with ammonia.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".

Bobbitt's salt is an oxoammonium compound derived from 4-acetamido-2,2,6,6-tetramethylpiperidine. It contains the tetrafluoroborate anion and is named after the American chemist James M. Bobbitt.

In chemistry, a Verkade base (or Verkade superbase) is a superbase with the formula P(MeNCH2CH2)3N. A colorless oil, it is an aminophosphine although its inventor John Verkade called it proazaphosphatrane. The trimethyl derivative or 2,5,8,9-tetraza-1-phosphabicyclo[3.3.3]undecane is the simplest. Diverse analogues of the Verkade base are known, e.g. with isopropyl groups in place of methyl.

Pinacolborane is the borane with the formula (CH3)4C2O2BH. Often pinacolborane is abbreviated HBpin. It features a boron hydride functional group incorporated in a five-membered C2O2B ring. Like related boron alkoxides, pinacolborane is monomeric. It is a colorless liquid. It features a reactive B-H functional group.

















