| |||
| Names | |||
|---|---|---|---|
| IUPAC names Dimethyl(pyridin-4-yl)azane Dimethyl(pyridin-4-yl)amine | |||
| Preferred IUPAC name N,N-Dimethylpyridin-4-amine | |||
| Other names 4-(Dimethylamino)pyridine N,N-Dimethyl-4-aminopyridine DMAP 4-Dimethylaminopyridine 4-(Dimethylamino)azine N,N-dimethyl-4-aminoazine 4-(Dimethylamino)azabenzene N,N-Dimethyl-4-aminoazabenzene | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.049 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C7H10N2 | |||
| Molar mass | 122.17 g/mol | ||
| Appearance | white solid | ||
| Melting point | 110 to 113 °C (230 to 235 °F; 383 to 386 K) | ||
| Boiling point | 162 °C (324 °F; 435 K) at 50 mmHg | ||
| Acidity (pKa) | 9.6 in water, 17.95 (pKa of conjugate acid in acetonitrile) [1] | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H301+H331, H310, H315, H319, H335 [2] | |||
| P280, P305+P351+P338, P337+P313 [2] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | deer mice: oral, 450 mg/kg [3] mice: oral, 350 mg/kg/day [3] Contents | ||
| Safety data sheet (SDS) | [2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
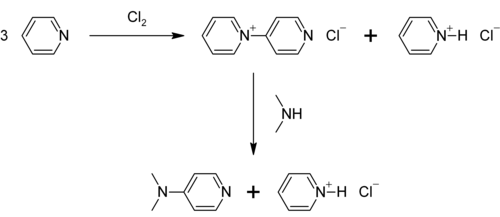
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH3)2NC5H4N. This white solid is of interest because it is more basic than pyridine, owing to the resonance stabilisation from the NMe2 substituent.
Because of its basicity, DMAP is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich rearrangement, Staudinger synthesis of β-lactams and many more. DMAP analogues are used in kinetic resolution experiments of mainly secondary alcohols and Evans auxiliary type amides. [4] [5] [6]