Plant hormones are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, from embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and through to reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.
Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. GAs are one of the longest-known classes of plant hormone. It is thought that the selective breeding of crop strains that were deficient in GA synthesis was one of the key drivers of the "green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide.

Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers.

Abscisic acid is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.

Plant senescence is the process of aging in plants. Plants have both stress-induced and age-related developmental aging. Chlorophyll degradation during leaf senescence reveals the carotenoids, such as anthocyanin and xanthophylls, which are the cause of autumn leaf color in deciduous trees. Leaf senescence has the important function of recycling nutrients, mostly nitrogen, to growing and storage organs of the plant. Unlike animals, plants continually form new organs and older organs undergo a highly regulated senescence program to maximize nutrient export.

Carotenoid oxygenases are a family of enzymes involved in the cleavage of carotenoids to produce, for example, retinol, commonly known as vitamin A. This family includes an enzyme known as RPE65 which is abundantly expressed in the retinal pigment epithelium where it catalyzed the formation of 11-cis-retinol from all-trans-retinyl esters.
Drought tolerance is the ability to which a plant maintains its biomass production during arid or drought conditions. Some plants are naturally adapted to dry conditions, surviving with protection mechanisms such as desiccation tolerance, detoxification, or repair of xylem embolism. Other plants, specifically crops like corn, wheat, and rice, have become increasingly tolerant to drought with new varieties created via genetic engineering. From an evolutionary perspective, the type of mycorrhizal associations formed in the roots of plants can determine how fast plants can adapt to drought.
In enzymology, a xanthoxin dehydrogenase (EC 1.1.1.288) is an enzyme that catalyzes the chemical reaction
Dehydrin (DHN) is a multi-family of proteins present in plants that is produced in response to cold and drought stress. DHNs are hydrophilic, reliably thermostable, and disordered. They are stress proteins with a high number of charged amino acids that belong to the Group II Late Embryogenesis Abundant (LEA) family. DHNs are primarily found in the cytoplasm and nucleus but more recently, they have been found in other organelles, like mitochondria and chloroplasts.

Phaseic acid is a terpenoid catabolite of abscisic acid. Like abscisic acid, it is a plant hormone associated with photosynthesis arrest and abscission.
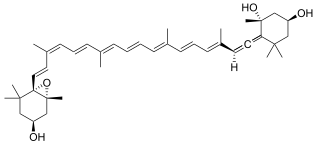
Neoxanthin is a carotenoid and xanthophyll. In plants, it is an intermediate in the biosynthesis of the plant hormone abscisic acid. It is often present in two forms: all-trans and 9-cis isomers. It is produced from violaxanthin, but a suspected neoxanthin synthase is still to be confirmed. Two different genes were confirmed to be implied in violaxanthin conversion to neoxanthin in Arabidopsis and tomato. It has a specific role in protection against photooxidative stress. It is a major xanthophyll found in green leafy vegetables such as spinach.
Zeaxanthin epoxidase (EC 1.14.13.90, Zea-epoxidase) is an enzyme with systematic name zeaxanthin,NAD(P)H:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction

Abscisic aldehyde is an intermediate in the biosynthesis of the plant hormone abscisic acid. It is produced by the dehydrogenation of xanthoxin by xanthoxin dehydrogenases, which is an NAD+ dependent short-chain dehydrogenase, followed by selective oxidation by abscisic aldehyde oxygenase.
WRKY transcription factors are proteins that bind DNA. They are transcription factors that regulate many processes in plants and algae (Viridiplantae), such as the responses to biotic and abiotic stresses, senescence, seed dormancy and seed germination and some developmental processes but also contribute to secondary metabolism.

Strigolactones are a group of chemical compounds produced by roots of plants. Due to their mechanism of action, these molecules have been classified as plant hormones or phytohormones. So far, strigolactones have been identified to be responsible for three different physiological processes: First, they promote the germination of parasitic organisms that grow in the host plant's roots, such as Strigalutea and other plants of the genus Striga. Second, strigolactones are fundamental for the recognition of the plant by symbiotic fungi, especially arbuscular mycorrhizal fungi, because they establish a mutualistic association with these plants, and provide phosphate and other soil nutrients. Third, strigolactones have been identified as branching inhibition hormones in plants; when present, these compounds prevent excess bud growing in stem terminals, stopping the branching mechanism in plants.
Jian-Kang Zhu is a plant scientist, researcher and academic. He is a Senior Principal Investigator in the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences (CAS). He is also the Academic Director of CAS Center of Excellence in Plant Sciences.
Hydraulic signals in plants are detected as changes in the organism's water potential that are caused by environmental stress like drought or wounding. The cohesion and tension properties of water allow for these water potential changes to be transmitted throughout the plant.
Barley is known to be more environmentally-tolerant than other cereal crops, in terms of soil pH, mineral nutrient availability, and water availability. Because of this, much research is being done on barley plants in order to determine whether or not there is a genetic basis for this environmental hardiness.
Christoph Benning is a German–American plant biologist. He is an MSU Foundation Professor and University Distinguished Professor at Michigan State University. Benning's research into lipid metabolism in plants, algae and photosynthetic bacteria, led him to be named Editor-in-Chief of The Plant Journal in October 2008.

Photoblasticism is a mechanism of seed dormancy. Photoblastic seeds require light in order to germinate. Once germination starts, the stored nutrients that have accumulated during maturation start to be digested which then supports cell expansion and overall growth. Within light-stimulated germination, Phytochrome B (PHYB) is the photoreceptor that is responsible for the beginning stages of germination. When red light is present, PHYB is converted to its active form and moves from the cytoplasm to the nucleus where it upregulates the degradation of PIF1. PIF1, phytochrome-interaction-factor-1, negatively regulates germination by increasing the expression of proteins that repress the synthesis of gibberellin (GA), a major hormone in the germination process. Another factor that promotes germination is HFR1 which accumulates in light in some way and forms inactive heterodimers with PIF1.