
A dendritic spine is a small membranous protrusion from a neuron's dendrite that typically receives input from a single axon at the synapse. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron's cell body. Most spines have a bulbous head, and a thin neck that connects the head of the spine to the shaft of the dendrite. The dendrites of a single neuron can contain hundreds to thousands of spines. In addition to spines providing an anatomical substrate for memory storage and synaptic transmission, they may also serve to increase the number of possible contacts between neurons. It has also been suggested that changes in the activity of neurons have a positive effect on spine morphology.

In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.

Pavlovian fear conditioning is a behavioral paradigm in which organisms learn to predict aversive events. It is a form of learning in which an aversive stimulus is associated with a particular neutral context or neutral stimulus, resulting in the expression of fear responses to the originally neutral stimulus or context. This can be done by pairing the neutral stimulus with an aversive stimulus. Eventually, the neutral stimulus alone can elicit the state of fear. In the vocabulary of classical conditioning, the neutral stimulus or context is the "conditional stimulus" (CS), the aversive stimulus is the "unconditional stimulus" (US), and the fear is the "conditional response" (CR).
An engram is a unit of cognitive information imprinted in a physical substance, theorized to be the means by which memories are stored as biophysical or biochemical changes in the brain or other biological tissue, in response to external stimuli.

Adult neurogenesis is the process in which neurons are generated from neural stem cells in the adult. This process differs from prenatal neurogenesis.

A place cell is a kind of pyramidal neuron in the hippocampus that becomes active when an animal enters a particular place in its environment, which is known as the place field. Place cells are thought to act collectively as a cognitive representation of a specific location in space, known as a cognitive map. Place cells work with other types of neurons in the hippocampus and surrounding regions to perform this kind of spatial processing. They have been found in a variety of animals, including rodents, bats, monkeys and humans.

Susumu Tonegawa is a Japanese scientist who was the sole recipient of the Nobel Prize for Physiology or Medicine in 1987 for his discovery of V(D)J recombination, the genetic mechanism which produces antibody diversity. Although he won the Nobel Prize for his work in immunology, Tonegawa is a molecular biologist by training and he again changed fields following his Nobel Prize win; he now studies neuroscience, examining the molecular, cellular and neuronal basis of memory formation and retrieval.

neurofibromatosis 1 (NF1) is a gene in humans that is located on chromosome 17. NF1 codes for neurofibromin, a GTPase-activating protein that negatively regulates RAS/MAPK pathway activity by accelerating the hydrolysis of Ras-bound GTP. NF1 has a high mutation rate and mutations in NF1 can alter cellular growth control, and neural development, resulting in neurofibromatosis type 1. Symptoms of NF1 include disfiguring cutaneous neurofibromas (CNF), café au lait pigment spots, plexiform neurofibromas (PN), skeletal defects, optic nerve gliomas, life-threatening malignant peripheral nerve sheath tumors (MPNST), pheochromocytoma, attention deficits, learning deficits and other cognitive disabilities.
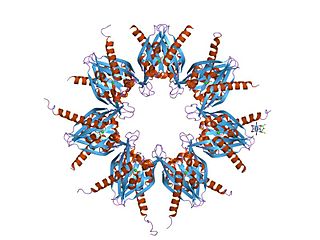
Ca2+
/calmodulin-dependent protein kinase II is a serine/threonine-specific protein kinase that is regulated by the Ca2+
/calmodulin complex. CaMKII is involved in many signaling cascades and is thought to be an important mediator of learning and memory. CaMKII is also necessary for Ca2+
homeostasis and reuptake in cardiomyocytes, chloride transport in epithelia, positive T-cell selection, and CD8 T-cell activation.
Molecular cellular cognition (MCC) is a branch of neuroscience that involves the study of cognitive processes with approaches that integrate molecular, cellular and behavioral mechanisms. Key goals of MCC studies include the derivation of molecular and cellular explanations of cognitive processes, as well as finding mechanisms and treatments for cognitive disorders.
Tobias Bonhoeffer is a German-American neurobiologist. He is director of the department Synapses – Circuits – Plasticity and current managing director at the Max Planck Institute for Biological Intelligence. His father, the neurobiologist Friedrich Bonhoeffer, was director at the Max Planck Institute for Developmental Biology in Tübingen.

Environmental enrichment is the stimulation of the brain by its physical and social surroundings. Brains in richer, more stimulating environments have higher rates of synaptogenesis and more complex dendrite arbors, leading to increased brain activity. This effect takes place primarily during neurodevelopment, but also during adulthood to a lesser degree. With extra synapses there is also increased synapse activity, leading to an increased size and number of glial energy-support cells. Environmental enrichment also enhances capillary vasculation, providing the neurons and glial cells with extra energy. The neuropil expands, thickening the cortex. Research on rodent brains suggests that environmental enrichment may also lead to an increased rate of neurogenesis.

Eric J. Nestler is the Nash Family Professor of Neuroscience, Director of the Friedman Brain Institute, and Dean for Academic Affairs at the Icahn School of Medicine at Mount Sinai and Chief Scientific Officer of the Mount Sinai Health System. His research is focused on a molecular approach to drug addiction and depression.
The cellular transcription factor CREB helps learning and the stabilization and retrieval of fear-based, long-term memories. This is done mainly through its expression in the hippocampus and the amygdala. Studies supporting the role of CREB in cognition include those that knock out the gene, reduce its expression, or overexpress it.
Memory allocation is a process that determines which specific synapses and neurons in a neural network will store a given memory. Although multiple neurons can receive a stimulus, only a subset of the neurons will induce the necessary plasticity for memory encoding. The selection of this subset of neurons is termed neuronal allocation. Similarly, multiple synapses can be activated by a given set of inputs, but specific mechanisms determine which synapses actually go on to encode the memory, and this process is referred to as synaptic allocation. Memory allocation was first discovered in the lateral amygdala by Sheena Josselyn and colleagues in Alcino J. Silva's laboratory.
While the cellular and molecular mechanisms of learning and memory have long been a central focus of neuroscience, it is only in recent years that attention has turned to the epigenetic mechanisms behind the dynamic changes in gene transcription responsible for memory formation and maintenance. Epigenetic gene regulation often involves the physical marking of DNA or associated proteins to cause or allow long-lasting changes in gene activity. Epigenetic mechanisms such as DNA methylation and histone modifications have been shown to play an important role in learning and memory.

Memory is commonly referred to as the ability to encode, store, retain and subsequently recall information and past experiences in the human brain. This process involves many proteins, one of which is the Histone-binding protein RbAp48, encoded by the RBBP4 gene in humans.
Hippocampal replay is a phenomenon observed in rats, mice, cats, rabbits, songbirds and monkeys. During sleep or awake rest, replay refers to the re-occurrence of a sequence of cell activations that also occurred during activity, but the replay has a much faster time scale. It may be in the same order, or in reverse. Cases were also found where a sequence of activations occurs before the actual activity, but it is still the same sequence. This is called preplay.
Attila Losonczy is a Hungarian neuroscientist, Professor of Neuroscience at Columbia University Medical Center. Losonczy's main area of research is on the relationship between neural networks and behavior, specifically with regard to learning in the hippocampus.











