
Frost is a thin layer of ice on a solid surface, which forms from water vapor in an above-freezing atmosphere coming in contact with a solid surface whose temperature is below freezing, and resulting in a phase change from water vapor to ice as the water vapor reaches the freezing point. In temperate climates, it most commonly appears on surfaces near the ground as fragile white crystals; in cold climates, it occurs in a greater variety of forms. The propagation of crystal formation occurs by the process of nucleation.

Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or 32 degrees Fahrenheit. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color.

Freezing, also known as solidification, is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid or the liquid content of a substance, usually due to cooling.
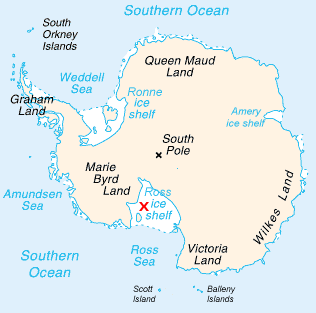
The Ross Ice Shelf is the largest ice shelf of Antarctica. It is several hundred metres thick. The nearly vertical ice front to the open sea is more than 600 kilometres (370 mi) long, and between 15 and 50 metres high above the water surface. Ninety percent of the floating ice, however, is below the water surface.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−55 °F). Droplets of supercooled water often exist in stratus and cumulus clouds. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes.

The underwater environment is the region below the surface of, and immersed in, liquid water in a natural or artificial feature, such as an ocean, sea, lake, pond, reservoir, river, canal, or aquifer. Some characteristics of the underwater environment are universal, but many depend on the local situation.

Sea ice arises as seawater freezes. Because ice is less dense than water, it floats on the ocean's surface. Sea ice covers about 7% of the Earth's surface and about 12% of the world's oceans. Much of the world's sea ice is enclosed within the polar ice packs in the Earth's polar regions: the Arctic ice pack of the Arctic Ocean and the Antarctic ice pack of the Southern Ocean. Polar packs undergo a significant yearly cycling in surface extent, a natural process upon which depends the Arctic ecology, including the ocean's ecosystems. Due to the action of winds, currents and temperature fluctuations, sea ice is very dynamic, leading to a wide variety of ice types and features. Sea ice may be contrasted with icebergs, which are chunks of ice shelves or glaciers that calve into the ocean. Depending on location, sea ice expanses may also incorporate icebergs.

An ice shelf is a large floating platform of ice that forms where a glacier or ice sheet flows down to a coastline and onto the ocean surface. Ice shelves are only found in Antarctica, Greenland, Northern Canada, and the Russian Arctic. The boundary between the floating ice shelf and the anchor ice that feeds it is the grounding line. The thickness of ice shelves can range from about 100 m (330 ft) to 1,000 m (3,300 ft).
In physics and chemistry, flash freezing is the process whereby objects are frozen in just a few hours by subjecting them to cryogenic temperatures, or through direct contact with liquid nitrogen at −196 °C (−320.8 °F). It is commonly used in the food industry.

Frazil ice is a collection of loose, randomly oriented ice crystals millimeter and sub-millimeter in size, with various shapes, e.g. elliptical disks, dendrites, needles and of an irregular nature. Frazil ice forms during the winter in open-water reaches of rivers as well as in lakes and reservoirs, where and when the water is in a turbulent state, which is, in turn, induced by the action of waves and currents. Turbulence causes the water column to become supercooled, as the heat exchange between the air and the water is such that the water temperature drops below its freezing point. The vertical mixing associated with that turbulence provides enough energy to overcome the crystals' buoyancy, thus keeping them from floating at the surface. Frazil ice also forms in oceans, where windy conditions, wave regimes and cold air also favor the establishment of a supercooled layer. Frazil ice can be found on the downwind side of leads, and in polynyas. In these environments, that ice can eventually accumulate at the water surface into what is referred to as grease ice.

Rime ice forms when supercooled water liquid droplets freeze onto surfaces. Meteorologists distinguish between three basic types of ice forming on vertical and horizontal surfaces by deposition of supercooled water droplets. There are also intermediate formations.

An ice spike is an ice formation, often in the shape of an inverted icicle, that projects upwards from the surface of a body of frozen water. Ice spikes created by natural processes on the surface of small bodies of frozen water have been reported for many decades, although their occurrence is quite rare. A mechanism for their formation, now known as the Bally–Dorsey model, was proposed in the early 20th century but this was not tested in the laboratory for many years. In recent years a number of photographs of natural ice spikes have appeared on the Internet as well as methods of producing them artificially by freezing distilled water in domestic refrigerators or freezers. This has allowed a small number of scientists to test the hypothesis in a laboratory setting and, although the experiments appear to confirm the validity of the Bally–Dorsey model, they have raised further questions about how natural ice spikes form, and more work remains to be done before the phenomenon is fully understood. Natural ice spikes can grow into shapes other than a classic spike shape, and have been variously reported as ice candles, ice towers or ice vases as there is no standard nomenclature for these other forms. One particularly unusual form takes the shape of an inverted pyramid.

Melt ponds are pools of open water that form on sea ice in the warmer months of spring and summer. The ponds are also found on glacial ice and ice shelves. Ponds of melted water can also develop under the ice.
Insect winter ecology describes the overwinter survival strategies of insects, which are in many respects more similar to those of plants than to many other animals, such as mammals and birds. Unlike those animals, which can generate their own heat internally (endothermic), insects must rely on external sources to provide their heat (ectothermic). Thus, insects persisting in winter weather must tolerate freezing or rely on other mechanisms to avoid freezing. Loss of enzymatic function and eventual freezing due to low temperatures daily threatens the livelihood of these organisms during winter. Not surprisingly, insects have evolved a number of strategies to deal with the rigors of winter temperatures in places where they would otherwise not survive.

Grease ice is a very thin, soupy layer of frazil crystals clumped together, which makes the ocean surface resemble an oil slick. Grease ice is the second stage in the formation of solid sea ice after ice floes and then frazil ice.

Ice sheet dynamics describe the motion within large bodies of ice, such those currently on Greenland and Antarctica. Ice motion is dominated by the movement of glaciers, whose gravity-driven activity is controlled by two main variable factors: the temperature and the strength of their bases. A number of processes alter these two factors, resulting in cyclic surges of activity interspersed with longer periods of inactivity, on both hourly and centennial time scales. Ice-sheet dynamics are of interest in modelling future sea level rise.

Polar seas is a collective term for the Arctic Ocean and the southern part of the Southern Ocean. In the coldest years, sea ice can cover around 13 percent of the Earth's total surface at its maximum, but out of phase in the two hemispheres. The polar seas contain a huge biome with many organisms.

Overdeepening is a characteristic of basins and valleys eroded by glaciers. An overdeepened valley profile is often eroded to depths which are hundreds of metres below the deepest continuous line along a valley or watercourse. This phenomenon is observed under modern day glaciers, in salt-water fjords and fresh-water lakes remaining after glaciers melt, as well as in tunnel valleys which are partially or totally filled with sediment. When the channel produced by a glacier is filled with debris, the subsurface geomorphic structure is found to be erosionally cut into bedrock and subsequently filled by sediments. These overdeepened cuts into bedrock structures can reach a depth of several hundred metres below the valley floor.

A brinicle is a downward-growing hollow tube of ice enclosing a plume of descending brine that is formed beneath developing sea ice.
Brine rejection is a process that occurs when salty water freezes. The salts do not fit in the crystal structure of water ice, so the salt is expelled.






















