Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.

Sodium hypochlorite, commonly known in a dilute solution as (chlorine) bleach, is an alkaline inorganic chemical compound with the formula NaOCl, consisting of a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

Hypochlorous acid is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.

Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivatives and chlorine. Alongside monochloramine and dichloramine, trichloramine is responsible for the distinctive 'chlorine smell' associated with swimming pools, where the compound is readily formed as a product from hypochlorous acid reacting with ammonia and other nitrogenous substances in the water, such as urea from urine.
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm.

Hypobromous acid is a weak, unstable acid with chemical formula of HOBr. It is mainly produced and handled in an aqueous solution. It is generated both biologically and commercially as a disinfectant. Salts of hypobromite are rarely isolated as solids.
Salt water chlorination is a process that uses dissolved salt for the chlorination of swimming pools and hot tubs. The chlorine generator uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms, hypochlorous acid and sodium hypochlorite, which are already commonly used as sanitizing agents in pools. Hydrogen is produced as byproduct too.

DBDMH is an organic compound derived from the heterocycle called dimethylhydantoin. This white crystalline compound with a slight bromine odor is widely used as a disinfectant used for drinking water purification, recreational water treatment, as a bleaching agent in pulp and paper mills, and for treating industrial/commercial water cooling systems. Its action does not involve the use of hypochlorous acid.
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting point of −66 °C (−87 °F), but it is usually handled as a dilute aqueous solution, in which form it is sometimes used as a disinfectant. Chloramine is too unstable to have its boiling point measured.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Electrolysed water is produced by the electrolysis of ordinary tap water containing dissolved sodium chloride. The electrolysis of such salt solutions produces a solution of hypochlorous acid and sodium hydroxide. The resulting water can be used as a disinfectant.

Swimming pool sanitation is the process of ensuring healthy conditions in swimming pools. Proper sanitation is needed to maintain the visual clarity of water and to prevent the transmission of infectious waterborne diseases.
Disinfection by-products (DBPs) are organic and inorganic compounds resulting from chemical reactions between organic and inorganic substances such as contaminates and chemical treatment disinfection agents, respectively, in water during water disinfection processes.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
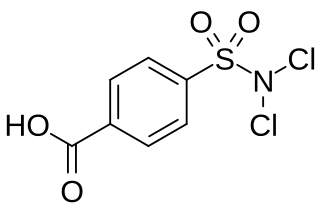
Halazone is a chemical compound whose formula can be written as either C
7H
5Cl
2NO
4S or (HOOC)(C
6H
4)(SO
2)(NCl
2). It has been widely used to disinfect drinking water.

Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid.
A mixed oxidant solution (MOS) is a type of disinfectant that has many uses including disinfecting, sterilizing, and eliminating pathogenic microorganisms in water. An MOS may have advantages such as a higher disinfecting power, stable residual chlorine in water, elimination of biofilm, and safety. The main components of an MOS are chlorine and its derivatives, which are produced by electrolysis of sodium chloride. It may also contain high amounts of hydroxy radicals, chlorine dioxide, dissolved ozone, hydrogen peroxide and oxygen from which the name "mixed oxidant" is derived.
A hypohalous acid is an oxyacid consisting of a hydroxyl group single-bonded to any halogen. Examples include hypofluorous acid, hypochlorous acid, hypobromous acid, and hypoiodous acid. The conjugate base is a hypohalite. They can be formed by reacting the corresponding diatomic halogen molecule with water in the reaction: