This article may be in need of reorganization to comply with Wikipedia's layout guidelines .(November 2018) |
The biochemistry of body odor pertains to the chemical compounds in the body responsible for body odor and their kinetics.
This article may be in need of reorganization to comply with Wikipedia's layout guidelines .(November 2018) |
The biochemistry of body odor pertains to the chemical compounds in the body responsible for body odor and their kinetics.
Body odor encompasses axillary (underarm) odor and foot odor. [1] It is caused by a combination of sweat gland secretions and normal skin microflora. [1] In addition, androstane steroids and the ABCC11 transporter are essential for most axillary odor. [1] [2] Body odor is a complex phenomenon, with numerous compounds and catalysts involved in its genesis. [1] Secretions from sweat glands are initially odorless, but preodoriferous compounds or malodor precursors in the secretions are transformed by skin surface bacteria into volatile odorous compounds that are responsible for body malodor. [1] [3] Water and nutrients secreted by sweat glands also contribute to body odor by creating an ideal environment for supporting the growth of skin surface bacteria. [1]
There are three types of sweat glands: eccrine, apocrine, and apoeccrine. [1] Apocrine glands are primarily responsible for body malodor and, along with apoeccrine glands, are mostly expressed in the axillary (underarm) regions, whereas eccrine glands are distributed throughout virtually all of the rest of the skin in the body, although they are also particularly expressed in the axillary regions, and contribute to malodor to a relatively minor extent. [1] Sebaceous glands, another type of secretory gland, are not sweat glands but instead secrete sebum (an oily substance), and may also contribute to body odor to some degree. [1]
The main odorous compounds that contribute to axillary odor include: [2]
These malodorous compounds are formed from non-odoriferous precursors that are secreted from apocrine glands and converted by various enzymes expressed in skin surface bacteria. [2] The specific skin surface bacteria responsible are mainly Staphylococcus and Corynebacterium species. [2]
The androstane steroids dehydroepiandrosterone sulfate (DHEA-S) and androsterone sulfate have been detected in an extract of axillary hairs together with high concentrations of cholesterol. [4] [1] Apocrine sweat contains relatively high amounts of androgens, for instance dehydroepiandrosterone (DHEA), androsterone, and testosterone, and the androgen receptor (AR), the biological target of androgens, is strongly expressed in the secretory cells of apocrine glands. [5] In addition, 5α-reductase type I, an enzyme which converts testosterone into the more potent androgen dihydrotestosterone (DHT), has been found to be highly expressed in the apocrine glands of adolescents, and DHT has been found to specifically contribute to malodor as well. [1] Starting at puberty, males have higher levels of androgens than do females and produce comparatively more axillary malodor. [5] As such, it has been proposed that the higher axillary malodor seen in males is due to greater relative stimulation of axillary apocrine sweat glands by androgens. [5]
ABCC11 is a gene encoding an apical ATP-driven efflux transporter that has been found to transport a variety of lipophilic anions including cyclic nucleotides, estradiol glucuronide, steroid sulfates such as DHEA-S, and monoanionic bile acids. [2] It is expressed and localized in apocrine glands, including in the axilla, the ceruminous glands in the auditory canal, and in the mammary gland. [2] A single-nucleotide polymorphism (SNP) 538G→A in ABCC11 that leads to a G180R substitution in the encoded protein has been found to result in loss-of-function via affecting N-linked glycosylation and in turn causing proteasomal degradation of the protein. [2] This polymorphism has been found to be responsible for the dry and white earwax phenotype, and is considered to be unique as it has been described as the only human SNP that has been found to determine a visible genetic trait. [2] In addition to earwax phenotype, the ABCC11 genotype has been found to be associated with colostrum secretion from the breasts as well as normal axillary odor and osmidrosis (excessive axillary malodor). [2]
A functional ABCC11 protein has been found to be essential for the presence of the characteristic strong axillary odor, with the 538G→A SNP leading to a loss of secretion of axillary malodorous precursors and a nearly complete loss of axillary odor in those who are homozygous for the polymorphism. [2] Specifically, the secretion of the amino-acid conjugates 3M2H-Gln, HMHA-Gln, and Cys-Gly-(S) 3M3SH, which are precursors of key axillary malodorous compounds including the unsaturated or hydroxylated branched-chain fatty acids 3M2H and HMHA and the sulfanylalkanol 3M3SH, has been found to be abolished in homozygotic carriers of the SNP, and the odoriferous androstane steroids androstenone and androstenol and their precursors DHEA and DHEA-S have been found to be significantly reduced as well. [2] Patients with axillary osmidrosis (538G/G or 538G/A genotype) were found to have significantly more numerous and relatively large axillary apocrine glands compared to controls with the A/A genotype. [3]
In contrast to the aforementioned odoriferous compounds, the levels of long straight-chain fatty acids such as hexadecanoic acid, octadecanoic acid, and linolic acid and short straight-chain fatty acids such as butyric acid, hexanoic acid, and octanoic acid in axillary sweat have not been found to be affected by the ABCC11 genotype, which suggests that their secretion is independent of ABCC11. [2] These straight-chain fatty acids are odoriferous, but differently and to a much lesser extent compared to branched-chain fatty acids. [2] In accordance, it has been said that it is very likely that these aliphatic straight-chain fatty acids are responsible for the faint sour acidic axillary odor that has previously been observed in most Japanese individuals. [2] In addition to the secretion of straight-chain fatty acids, axillary microflora did not appear to differ between homozygous carriers of the 538G→A SNP and non-carriers. [2]
The ABCC11 transporter appears to be involved both in the transport of androstane steroids into the secretory cells of apocrine glands and in the secretion of preodoriferous compounds from axillary apocrine glands. [3] Specific steroids that ABCC11 has been found to transport include steroid sulfates like DHEA-S and estrone sulfate and steroid glucuronides like estradiol glucuronide. [4] In accordance with its transport of compounds involved in axillary odor, ABCC11 alleles are strongly associated with axillary odor. [3] Asians have little or faint axillary odor, whereas Caucasians and Africans have strong axillary odor, and this has been found to be due to genetic differences in the ABCC11 gene. [3]
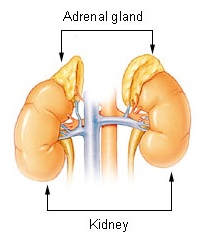
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.

In animals, a gland is a group of cells in an animal's body that synthesizes substances for release into the bloodstream or into cavities inside the body or its outer surface.

The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.
A deodorant is a substance applied to the body to prevent or mask body odor due to bacterial breakdown of perspiration or vaginal secretions, for example in the armpits, groin, or feet. A subclass of deodorants, called antiperspirants, prevents sweating itself, typically by blocking sweat glands. Antiperspirants are used on a wider range of body parts, at any place where sweat would be inconvenient or unsafe, since unwanted sweating can interfere with comfort, vision, and grip. Other types of deodorant allow sweating but prevent bacterial action on sweat, since human sweat only has a noticeable smell when it is decomposed by bacteria.

A sebaceous gland, or oil gland, is a microscopic exocrine gland in the skin that opens into a hair follicle to secrete an oily or waxy matter, called sebum, which lubricates the hair and skin of mammals. In humans, sebaceous glands occur in the greatest number on the face and scalp, but also on all parts of the skin except the palms of the hands and soles of the feet. In the eyelids, meibomian glands, also called tarsal glands, are a type of sebaceous gland that secrete a special type of sebum into tears. Surrounding the female nipple, areolar glands are specialized sebaceous glands for lubricating the nipple. Fordyce spots are benign, visible, sebaceous glands found usually on the lips, gums and inner cheeks, and genitals.
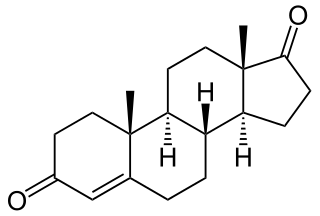
Androstenedione, or 4-androstenedione, also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroepiandrosterone (DHEA). It is closely related to androstenediol (androst-5-ene-3β,17β-diol).
Body odor or body odour (BO) is present in all animals and its intensity can be influenced by many factors. Body odor has a strong genetic basis, but can also be strongly influenced by various factors, such as gender, diet, health, and medication. The body odor of human males plays an important role in human sexual attraction, as a powerful indicator of MHC/HLA heterozygosity. Significant evidence suggests that women are attracted to men whose body odor is different from theirs, indicating that they have immune genes that are different from their own, which may produce healthier offspring.
Adrenarche is an early stage in sexual maturation that happens in some higher primates and in humans, typically peaks at around 20 years of age, and is involved in the development of pubic hair, body odor, skin oiliness, axillary hair, sexual attraction/sexual desire/increased libido and mild acne. During adrenarche the adrenal glands secrete increased levels of weak adrenal androgens, including dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione (A4), but without increased cortisol levels. Adrenarche is the result of the development of a new zone of the adrenal cortex, the zona reticularis. Adrenarche is a process related to puberty, but distinct from hypothalamic–pituitary–gonadal axis maturation and function.

Sweat glands, also known as sudoriferous or sudoriparous glands, from Latin sudor 'sweat', are small tubular structures of the skin that produce sweat. Sweat glands are a type of exocrine gland, which are glands that produce and secrete substances onto an epithelial surface by way of a duct. There are two main types of sweat glands that differ in their structure, function, secretory product, mechanism of excretion, anatomic distribution, and distribution across species:

Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.

Androstenol, also known as 5α-androst-16-en-3α-ol, is a 16-androstene class steroidal pheromone and neurosteroid in humans and other mammals, notably pigs. It possesses a characteristic musk-like odor.
An apocrine sweat gland is composed of a coiled secretory portion located at the junction of the dermis and subcutaneous fat, from which a straight portion inserts and secretes into the infundibular portion of the hair follicle. In humans, apocrine sweat glands are found only in certain locations of the body: the axillae (armpits), areola and nipples of the breast, ear canal, eyelids, wings of the nostril, perineal region, and some parts of the external genitalia. Modified apocrine glands include the ciliary glands in the eyelids; the ceruminous glands, which produce ear wax; and the mammary glands, which produce milk. The rest of the body is covered by eccrine sweat glands.
Fox–Fordyce disease is a chronic blockage of the sweat gland ducts with a secondary, non-bacterial inflammatory response to the secretions and cellular debris in the cysts. The inflammation is often accompanied by intense itching. In general, the disease often causes skin to darken near the affected area and raised bumps or papules to appear. In addition, hair follicles can become damaged which cause hair loss. Hidradenitis is very similar, but tends to have a secondary bacterial infection so that pus-draining sinuses are formed. It is a very devastating skin disease that does not have universally curative treatments.
Skin appendages are anatomical skin-associated structures that serve a particular function including sensation, contractility, lubrication and heat loss in animals. In humans, some of the more common skin appendages are hairs, arrector pilli, sebaceous glands, sweat glands, and nails (protection).

ATP-binding cassette transporter sub-family C member 11, also MRP8 is a membrane transporter that exports certain molecules from inside a cell. It is a protein that in humans is encoded by gene ABCC11.
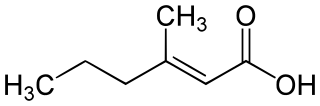
trans-3-Methyl-2-hexenoic acid (TMHA) is an unsaturated short-chain fatty acid that occurs in sweat secreted by the axillary (underarm) apocrine glands of Caucasians and some Asians.

16-Androstenes, or androst-16-enes, are a class of endogenous androstane steroids that includes androstadienol, androstadienone, androstenone, and androstenol, which are pheromones. Some of the 16-androstenes, such as androstenone and androstenol, are odorous, and have been confirmed to contribute to human malodor.
Adrenal steroids are steroids that are derived from the adrenal glands. They include corticosteroids, which consist of glucocorticoids like cortisol and mineralocorticoids like aldosterone, adrenal androgens like dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), and androstenedione (A4), and neurosteroids like DHEA and DHEA-S, as well as pregnenolone and pregnenolone sulfate (P5-S). Adrenal steroids are specifically produced in the adrenal cortex.

Etiocholanedione, also known as 5β-androstanedione or as etiocholane-3,17-dione, is a naturally occurring etiocholane (5β-androstane) steroid and an endogenous metabolite of androgens like testosterone, dihydrotestosterone, dehydroepiandrosterone (DHEA), and androstenedione. It is the C5 epimer of androstanedione (5α-androstanedione). Although devoid of androgenic activity like other 5β-reduced steroids, etiocholanedione has some biological activity of its own. The compound has been found to possess potent haematopoietic effects in a variety of models. In addition, it has been found to promote weight loss in animals and in a double-blind, placebo-controlled clinical study in humans conducted in 1993. These effects are said to be similar to those of DHEA. Unlike DHEA however, etiocholanedione cannot be metabolized further into steroid hormones like androgens and estrogens.