
Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Artemisia annua, also known as sweet wormwood, sweet annie, sweet sagewort, annual mugwort or annual wormwood, is a common type of wormwood native to temperate Asia, but naturalized in many countries including scattered parts of North America.
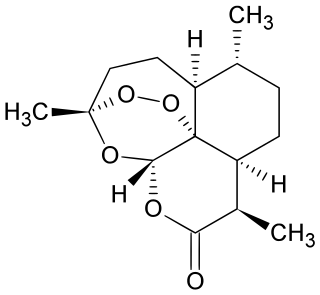
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua, sweet wormwood, a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
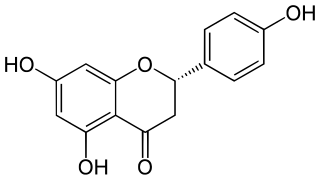
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

Rutin, also called rutoside, quercetin-3-O-rutinoside and sophorin, is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid found in a wide variety of plants, including citrus.

Naringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.

Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an endo position. The exo diastereomer is called isoborneol. Being chiral, borneol exists as enantiomers, both of which are found in nature.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.

Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one).

Pinocembrin is a flavanone, a type of flavonoid. It is an antioxidant found in damiana, honey, fingerroot, and propolis.
In enzymology, a flavanone 3-dioxygenase (EC 1.14.11.9) is an enzyme that catalyzes the chemical reaction

Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.
The enzyme amorpha-4,11-diene synthase (ADS) catalyzes the chemical reaction

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone, a neurotoxin also found in Artemisia absinthium.
The O-methylated flavonoids or methoxyflavonoids are flavonoids with methylations on hydroxyl groups. O-methylation has an effect on the solubility of flavonoids.

Blumea balsamifera is a flowering plant belonging to the genus Blumea of the family Asteraceae. It is also known as Ngai camphor and sambong.
Flavonoid 3',5'-hydroxylase (EC 1.14.14.81 was wrongly classified as EC 1.14.13.88 in the past) is an enzyme with systematic name flavanone,NADPH:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction

Casticin is a methoxylated flavonol, meaning the core flavonoid structure has methyl groups attached. Found in Artemisia annua, the flavonoid has been shown to enhance the antimalarial activity of artemisinin though casticin itself has no direct antimalarial effects. It has been shown to have anti-mitotic activity. It is also found in Vitex agnus-castus.
Pseudonocardia xishanensis is a Gram-positive and non-motile bacterium from the genus of Pseudonocardia which has been isolated from the roots of the plant Artemisia annua from the Xishan Mountains in China.















