Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

In biochemistry, ABTS is a chemical compound used to observe the reaction kinetics of specific enzymes. A common use for it is in the enzyme-linked immunosorbent assay (ELISA) to detect the binding of molecules to each other.

Barbital, marketed under the brand names Veronal for the pure acid and Medinal for the sodium salt, was the first commercially available barbiturate. It was used as a sleeping aid (hypnotic) from 1903 until the mid-1950s. The chemical names for barbital are diethylmalonyl urea or diethylbarbituric acid; hence, the sodium salt is known also as sodium diethylbarbiturate.
Grapefruit seed extract (GSE), also known as citrus seed extract, is a liquid extract derived from the seeds, pulp, and white membranes of grapefruit. GSE is prepared by grinding the grapefruit seed and juiceless pulp, then mixing with glycerin. Commercially available GSEs sold to consumers are made from the seed, pulp, and glycerin blended together. GSE is sold as a dietary supplement and is used in cosmetics.
Hydroxycinnamic acids (hydroxycinnamates) are a class of aromatic acids or phenylpropanoids having a C6–C3 skeleton. These compounds are hydroxy derivatives of cinnamic acid.

The Folin–Ciocâlteu reagent (FCR) or Folin's phenol reagent or Folin–Denis reagent, is a mixture of phosphomolybdate and phosphotungstate used for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants, also called the gallic acid equivalence method (GAE). It is named after Otto Folin, Vintilă Ciocâlteu, and Willey Glover Denis. The Folin-Denis reagent is prepared by mixing sodium tungstate and phosphomolybdic acid in phosphoric acid. The Folin–Ciocalteu reagent is just a modification of the Folin-Denis reagent. The modification consisted of the addition of lithium sulfate and bromine to the phosphotungstic-phosphomolybdic reagent.

Eriodictyon californicum is a species of plant within the family Boraginaceae. It is also known as yerba santa, mountain balm, bear's weed, gum bush, gum plant, and consumptive weed. Less common names include Herbe des Montagnes, Herbe à Ourse, Herbe Sacrée, Herbe Sainte, Hierba Santa, Holy Herb, and Tarweed.

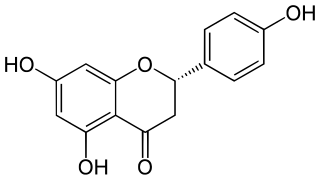
Eriodictyol is a bitter-masking flavanone, a flavonoid extracted from yerba santa, a plant native to North America. Eriodictyol is one of the four flavanones identified in this plant as having taste-modifying properties, the other three being homoeriodictyol, its sodium salt, and sterubin.

Sterubin (7-methoxy-3',4',5-trihydroxyflavanone) is a bitter-masking flavanone extracted from Yerba Santa a plant growing in America.
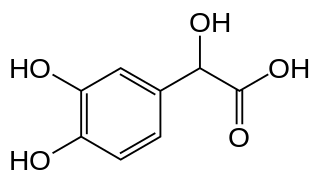
3,4-Dihydroxymandelic acid is a metabolite of norepinephrine.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

Eriodictyon parryi or poodle-dog bush is a tall California mountain shrub with showy purple flowers, which is notable for secreting a severe skin irritant. It is an opportunistic species that grows mostly in areas that have been disturbed by fire. In a dry early spring in Southern California, its semi-dormant leaves can droop and curl into coils like locks of curly hair, hence the popular name based on the metaphor of a poodle's natural hair.

Astilbin is a flavanonol, a type of flavonoid. Astilbin is the (2R-trans)-isomer; neoisoastilbin is the (2S-cis)-isomer and isoastilbin is the (2R-cis)-isomer.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

The grape reaction product is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing. It is also found in aged red wines. Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts, especially in white wine production. The product can be recreated in model solutions.
2,4-Dihydroxybenzoic acid is a dihydroxybenzoic acid.

6-Methoxymellein is a dihydroisocoumarin, a phenolic compound found in carrots and carrot purées. It is responsible for bitterness in carrots. It is a phytoalexin, induced in carrot slices by UV-C, that allows resistance to Botrytis cinerea and other microorganisms.

Melitidin is a flavanone glycoside. Melitidin was discovered in bergamot orange juice and exhibits statin-like properties so the juice seems to have hypolipidemic activity.

Brutieridin is a flavanone glycoside. Brutieridin was discovered in bergamot orange juice and exhibits statin-like properties as well as an anticholesterolaemic effect. As a result, the juice seems to have hypolipidemic (lipid-lowering) activity.


















