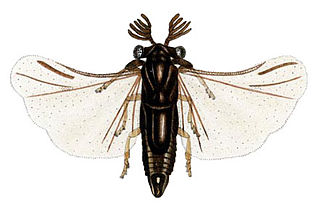
The Strepsiptera are an order of insects with eleven extant families that include about 600 described species. They are endoparasites of other insects, such as bees, wasps, leafhoppers, silverfish, and cockroaches. Females of most species never emerge from the host after entering its body, finally dying inside it. The early-stage larvae do emerge because they must find an unoccupied living host, and the short-lived males must emerge to seek a receptive female in her host. They are believed to be most closely related to beetles, from which they diverged 300–350 million years ago, but do not appear in the fossil record until the mid-Cretaceous around 100 million years ago.

An instar is a developmental stage of arthropods, such as insects, between each moult (ecdysis), until sexual maturity is reached. Arthropods must shed the exoskeleton in order to grow or assume a new form. Differences between instars can often be seen in altered body proportions, colors, patterns, changes in the number of body segments or head width. After shedding their exoskeleton (moulting), the juvenile arthropods continue in their life cycle until they either pupate or moult again. The instar period of growth is fixed; however, in some insects, like the salvinia stem-borer moth, the number of instars depends on early larval nutrition. Some arthropods can continue to moult after sexual maturity, but the stages between these subsequent moults are generally not called instars.

The common blue butterfly or European common blue is a butterfly in the family Lycaenidae and subfamily Polyommatinae. The butterfly is found throughout the Palearctic and has been introduced to North America. Butterflies in the Polyommatinae are collectively called blues, from the coloring of the wings. Common blue males usually have wings that are blue above with a black-brown border and a white fringe. The females are usually brown above with a blue dusting and orange spots.

Manduca sexta is a moth of the family Sphingidae present through much of the Americas. The species was first described by Carl Linnaeus in his 1763 Centuria Insectorum.

Polygonia c-album, the comma, is a food generalist (polyphagous) butterfly species belonging to the family Nymphalidae. The angular notches on the edges of the forewings are characteristic of the genus Polygonia, which is why species in the genus are commonly referred to as anglewing butterflies. Comma butterflies can be identified by their prominent orange and dark brown/black dorsal wings.

The Phoridae are a family of small, hump-backed flies resembling fruit flies. Phorid flies can often be identified by their escape habit of running rapidly across a surface rather than taking to the wing. This behaviour is a source of one of their alternate names, scuttle fly. Another vernacular name, coffin fly, refers to Conicera tibialis. About 4,000 species are known in 230 genera. The most well-known species is cosmopolitan Megaselia scalaris. At 0.4 mm in length, the world's smallest fly is the phorid Euryplatea nanaknihali.
Gnathostoma spinigerum is a parasitic nematode that causes gnathostomiasis in humans, also known as its clinical manifestations are creeping eruption, larva migrans, Yangtze edema, Choko-Fuschu Tua chid and wandering swelling. Gnathostomiasis in animals can be serious, and even fatal. The first described case of gnathostomiasis was in a young tiger that died in the London Zoo in 1835. The larval nematode is acquired by eating raw or undercooked fish and meat.

Myrmecolacidae is an insect family of the order Strepsiptera. There are four genera and about 98 species in this family. Like all strepsipterans, they have a parasitic mode of development with males parasitizing ants while the females develop inside Orthoptera. The sexes differ greatly in morphology making it very difficult to match females to the better catalogued museum specimens of males.

The Mediterranean flour moth or mill moth is a moth of the family Pyralidae. It is a common pest of cereal grains, especially flour. This moth is found throughout the world, especially in countries with temperate climates. It prefers warm temperatures for more rapid development, but it can survive a wide range of temperatures.

Phengaris rebeli, common name mountain Alcon blue, is a species of butterfly in the family Lycaenidae. It was first found and described in Styria, Austria, on Mount Hochschwab around 1700. Although it was initially classified as a subspecies of P. alcon, a European researcher, Lucien A. Berger, designated it as a separate species in 1946. Genetic similarities between P. rebeli and P. alcon have led many researchers to argue that the two are the same species and differences are due to intraspecific variation.
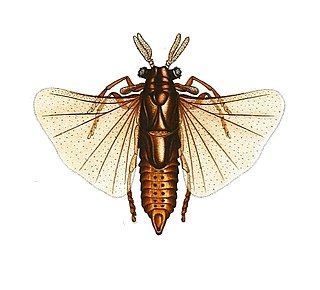
Xenos vesparum is a parasitic insect species of the order Strepsiptera that are endoparasites of paper wasps in the genus Polistes that was first described in 1793. Like other members of this family, X. vesparum displays a peculiar lifestyle, and demonstrates extensive sexual dimorphism.

Jalmenus evagoras, the imperial hairstreak, imperial blue, or common imperial blue, is a small, metallic blue butterfly of the family Lycaenidae. It is commonly found in eastern coastal regions of Australia. This species is notable for its unique mutualism with ants of the genus Iridomyrmex. The ants provide protection for juveniles and cues for adult mating behavior. They are compensated with food secreted from J. evagoras larvae. The ants greatly enhance the survival and reproductive success of the butterflies. J. evagoras lives and feeds on Acacia plants, so butterfly populations are localized to areas with preferred species of both host plants and ants.
Bucolus fourneti is a native Australian, small, hairy coccinellid beetle approximately 2.1-4.5 mm in diameter. It was described by Étienne Mulsant in 1850

Eurybia elvina, commonly known as the blind eurybia, is a Neotropical metalmark butterfly. Like many other riodinids, the caterpillars are myrmecophilous and have tentacle nectary organs that exude a fluid similar to that produced by the host plant Calathea ovandensis. This mutualistic relationship allows ants to harvest the exudate, and in return provide protection in the form of soil shelters for larvae. The larvae communicate with the ants by vibrations produced by the movement of its head. The species was described and given its binomial name by the German lepidopterist Hans Stichel in 1910.

Hemileuca lucina, the New England buck moth, is a species of moth in the family Saturniidae. This moth species is only found in the New England region of the United States. Larvae in early stages mainly feed on broadleaf meadowsweet whereas larvae in later stages show variation in food sources such as blackberry and black cherry leaves. Larvae have a black body with orange/black spines on their back that are used to deter predators. Pupation occurs during the summer and adult moths come out around September.
Glyphidops flavifrons is a member of the Neriidae family of the order Diptera. This fly is found in the southern United States, Central America, and South America. Historically, it has also been called Oncopsia seductrix Hennig or Oncopsia mexicana.G. flavifrons live, reproduce, and lay their eggs on the bark of trees in the early stages of decay. In this species, it is common to see the male flies to exhibit aggression in the presence of the females. These males may attack the copulating pair to help decrease the chances of other males mating and increase their own chances.
Pseudacteon tricuspis is a parasitic phorid fly that decapitates its host, the imported Solenopsis invicta fire ant. There are over 70 described species within the Pseudacteon genus, which parasitize a variety of ant species. However, P. tricuspis is very specific to its host ant and will not attack other native ant species, making it a good biological control against the fire ant. P. tricuspis was also introduced into the United States for this purpose. Aside from the United States, P. tricuspis has also been found in South America, Europe, and Asia. Female P. tricuspis deposit their eggs directly into the fire ant host. Deposition into the ant host determines the sex of the egg, which grows within the host until adulthood, killing and decapitating the host in the process. Interestingly, P. tricuspis has a male-biased sex ratio, where the males are smaller than the females.
Abscondita chinensis, is a species of firefly beetle found in India, China and Sri Lanka.
Silana farinosa, commonly known as curry-leaf tortoise beetle, is a species of leaf beetle native to Indo-China, India, Sri Lanka, Thailand and introduced to Peninsular Malaysia.
Halictoxenos borealis is a species of the order Strepsiptera of flying insects, that parasitize sweat bees (Lasioglossum).













