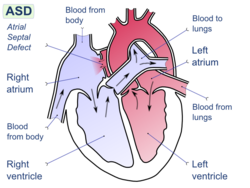
Biological
Some acquired shunts are modifications of congenital ones: a balloon septostomy can enlarge a foramen ovale (if performed on a newborn), PFO or ASD; or prostaglandin can be administered to a newborn to prevent the ductus arteriosus from closing. Biological tissues may also be used to construct artificial passages.
Evaluation can be done during a cardiac catheterization with a "shunt run" by taking blood samples from superior vena cava (SVC), inferior vena cava (IVC), right atrium, right ventricle, pulmonary artery, and system arterial. Abrupt increases in oxygen saturation support a left-to-right shunt and lower than normal systemic arterial oxygen saturation supports a right-to-left shunt.
Samples from the SVC & IVC are used to calculate mixed venous oxygen saturation using the Flamm formula

and Qp:Qs ratio

where  is the pulmonary vein,
is the pulmonary vein,  is the pulmonary artery,
is the pulmonary artery,  is the systemic arterial, and
is the systemic arterial, and  is the mixed-venous The Qp:Qs ratio is based upon the Fick principle and it is reduced to the above equation and eliminates the need to know cardiac output and hemoglobin concentration.
is the mixed-venous The Qp:Qs ratio is based upon the Fick principle and it is reduced to the above equation and eliminates the need to know cardiac output and hemoglobin concentration.