 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Chloro(methoxy)methane | |
| Other names MOM-Cl, CMME, MCD, Chlorodimethyl ether, Chloromethoxymethane, Dimethylchloroether, Methylchloromethyl ether | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.003.165 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1239 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C2H5ClO | |
| Molar mass | 80.51 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Irritating and acrid |
| Density | 1.06 g/mL |
| Melting point | −103.5 °C (−154.3 °F; 169.7 K) |
| Boiling point | 55–57 °C (131–135 °F; 328–330 K) |
| reacts | |
| Solubility | Soluble in alcohol and diethylether |
| Vapor pressure | 192 mmHg (21°C) [2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Carcinogen & Irritant |
| GHS labelling: | |
   | |
| Danger | |
| H225, H302, H312, H319, H332, H350 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P322, P330, P337+P313, P363, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 0 °C (32 °F; 273 K) (open cup) [2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | OSHA-Regulated Carcinogen, no PEL [2] |
REL (Recommended) | Carcinogenic [2] |
IDLH (Immediate danger) | N.D. [2] |
| Safety data sheet (SDS) | Safety Data Sheet Archived |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
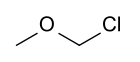
Chloromethyl methyl ether (CMME) is a compound with formula CH3OCH2Cl. A colorless liquid, it is a chloroalkyl ether. It is used as an alkylating agent. In organic synthesis, it is used for introducing the methoxymethyl ether (MOM) protecting group, [3] and is thus often called MOM-Cl or MOM chloride. It also finds application as a chloromethylating agent in some variants of the Blanc chloromethylation. [4]
