Related Research Articles

Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases, that arise from electromagnetic forces between atoms and electrons. More generally, the subject deals with condensed phases of matter: systems of many constituents with strong interactions among them. More exotic condensed phases include the superconducting phase exhibited by certain materials at extremely low cryogenic temperatures, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, the Bose–Einstein condensates found in ultracold atomic systems, and liquid crystals. Condensed matter physicists seek to understand the behavior of these phases by experiments to measure various material properties, and by applying the physical laws of quantum mechanics, electromagnetism, statistical mechanics, and other physics theories to develop mathematical models and predict the properties of extremely large groups of atoms.

Glass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass like "a glass" of water, "glasses", and "magnifying glass", are named after the material.

Tungsten is a chemical element; it has symbol W and atomic number 74. It is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternative name.

Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, brittle material, normally classified as a ceramic in its pure form, and is a frequently found and important constituent in ferrous metallurgy. While cementite is present in most steels and cast irons, it is produced as a raw material in the iron carbide process, which belongs to the family of alternative ironmaking technologies. The name cementite originated from the theory of Floris Osmond and J. Werth, in which the structure of solidified steel consists of a kind of cellular tissue, with ferrite as the nucleus and Fe3C the envelope of the cells. The carbide therefore cemented the iron.

Group 12, by modern IUPAC numbering, is a group of chemical elements in the periodic table. It includes zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn). Formerly this group was named IIB by CAS and old IUPAC system.
The original Tristan chord is heard in the opening phrase of Richard Wagner's opera Tristan und Isolde as part of the leitmotif relating to Tristan. It is made up of the notes F, B, D♯, and G♯:

Cocoa butter, also called theobroma oil, is a pale-yellow, edible fat extracted from the cocoa bean. It is used to make chocolate, as well as some ointments, toiletries, and pharmaceuticals. Cocoa butter has a cocoa flavor and aroma. Its melting point is slightly below human body temperature. It is an essential ingredient of chocolate and related confectionary products. Cocoa butter does not contain butter or other animal products; it is vegan.
The bond-dissociation energy is one measure of the strength of a chemical bond A−B. It can be defined as the standard enthalpy change when A−B is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K, although the enthalpy change at 298 K is also a frequently encountered parameter.

Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula Ta
2O
5. It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. Ta
2O
5 is an inert material with a high refractive index and low absorption, which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant.

Chocolate ice cream is ice cream with natural or artificial chocolate flavoring. One of the oldest flavours of ice creams, it is also one of the world's most popular. While most often sold alone, it is also a base for many other flavours.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The three main categories are potentiometry, amperometry, coulometry.
In queueing models, a discipline within the mathematical theory of probability, the quasi-birth–death process describes a generalisation of the birth–death process. As with the birth-death process it moves up and down between levels one at a time, but the time between these transitions can have a more complicated distribution encoded in the blocks.
Supercritical adsorption also referred to as the adsorption of supercritical fluids, is the adsorption at above-critical temperatures. There are different tacit understandings of supercritical fluids. For example, “a fluid is considered to be ‘supercritical’ when its temperature and pressure exceed the temperature and pressure at the critical point”. In the studies of supercritical extraction, however, “supercritical fluid” is applied for a narrow temperature region of 1-1.2 or to +10 K, which is called the supercritical region.
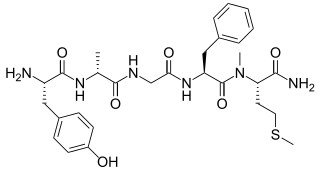
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.

Nickel sulfide is any inorganic compound with the formula NixSy. These compounds range in color from bronze (Ni3S2) to black (NiS2). The nickel sulfide with simplest stoichiometry is NiS, also known as the mineral millerite. From the economic perspective, Ni9S8, the mineral pentlandite, is the chief source of mined nickel. Other minerals include heazlewoodite (Ni3S2) and polydymite (Ni3S4), and the mineral Vaesite (NiS2). Some nickel sulfides are used commercially as catalysts.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally used in this article.
Richard Robson is a Professor of Chemistry at the University of Melbourne. Robson has published over 200 articles, specialising in coordination polymers, particularly metal-organic frameworks. He has been described as "a pioneer in crystal engineering involving transition metals".
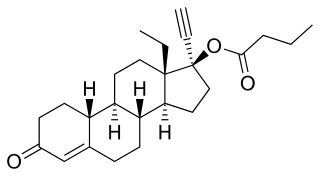
Levonorgestrel butanoate (LNG-B), or levonorgestrel 17β-butanoate, is a steroidal progestin of the 19-nortestosterone group which was developed by the World Health Organization (WHO) in collaboration with the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development as a long-acting injectable contraceptive. It is the C17β butanoate ester of levonorgestrel, and acts as a prodrug of levonorgestrel in the body. The drug is at or beyond the phase III stage of clinical development, but has not been marketed at this time. It was first described in the literature, by the WHO, in 1983, and has been under investigation for potential clinical use since then.

Oiticica oil is a light-yellowish oil obtained from the seeds of oiticica tree which grows mainly in Brazil.
References
- ↑ Padley, Fred B.; Fred B. Padley (1997). Lipid technologies and applications. CRC Press. p. 397. ISBN 978-0-8247-9838-3 . Retrieved 27 August 2010.
- ↑ Hartel, Richard W. (1998). "Phase Transitions in Chocolate and Coatings". In M. A. Rao, Richard W. Hartel (ed.). Phase/state transitions in foods: chemical, structural, and rheological changes. CRC Press. pp. 217–52. ISBN 978-0-8247-0179-6 . Retrieved 27 August 2010.
- ↑ Manley, Duncan J. R. (1998). Secondary processing in biscuit manufacturing: chocolate enrobing, moulding, sandwich creaming, icing, application of jam, marshmallow, caramel, troubleshooting tips, Volume 4. Woodhead. ISBN 978-1-85573-296-4.