Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C4H9OH, which occurs in five isomeric structures (four structural isomers), from a straight-chain primary alcohol to a branched-chain tertiary alcohol; all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, n-BuOH, i-BuOH, and t-BuOH). These are n-butanol, 2 stereoisomers of sec-butanol, isobutanol and tert-butanol. Butanol is primarily used as a solvent and as an intermediate in chemical synthesis, and may be used as a fuel. Biologically produced butanol is called biobutanol, which may be n-butanol or isobutanol.

The Clostridia are a highly polyphyletic class of Bacillota, including Clostridium and other similar genera. They are distinguished from the Bacilli by lacking aerobic respiration. They are obligate anaerobes and oxygen is toxic to them. Species of the class Clostridia are often but not always Gram-positive and have the ability to form spores. Studies show they are not a monophyletic group, and their relationships are not entirely certain. Currently, most are placed in a single order called Clostridiales, but this is not a natural group and is likely to be redefined in the future.

Clostridium acetobutylicum, ATCC 824, is a commercially valuable bacterium sometimes called the "Weizmann Organism", after Jewish Russian-born biochemist Chaim Weizmann. A senior lecturer at the University of Manchester, England, he used them in 1916 as a bio-chemical tool to produce at the same time, jointly, acetone, ethanol, and n-butanol from starch. The method has been described since as the ABE process,, yielding 3 parts of acetone, 6 of n-butanol, and 1 of ethanol. Acetone was used in the important wartime task of casting cordite. The alcohols were used to produce vehicle fuels and synthetic rubber.

1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C4H9OH and a linear structure. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol. The unmodified term butanol usually refers to the straight chain isomer.

Butanol may be used as a fuel in an internal combustion engine. It is more similar to gasoline than it is to ethanol. A C4-hydrocarbon, butanol is a drop-in fuel and thus works in vehicles designed for use with gasoline without modification. Both n-butanol and isobutanol have been studied as possible fuels. Both can be produced from biomass (as "biobutanol" ) as well as from fossil fuels (as "petrobutanol"). The chemical properties depend on the isomer (n-butanol or isobutanol), not on the production method.
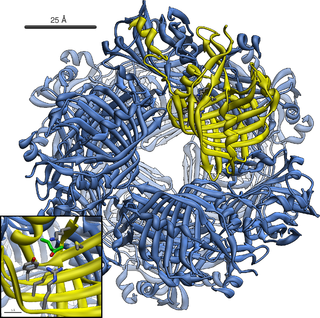
Acetoacetate decarboxylase is an enzyme involved in both the ketone body production pathway in humans and other mammals, and solventogenesis in bacteria. Acetoacetate decarboxylase plays a key role in solvent production by catalyzing the decarboxylation of acetoacetate, yielding acetone and carbon dioxide.

Acetone–butanol–ethanol (ABE) fermentation, also known as the Weizmann process, is a process that uses bacterial fermentation to produce acetone, n-butanol, and ethanol from carbohydrates such as starch and glucose. It was developed by chemist Chaim Weizmann and was the primary process used to produce acetone, which was needed to make cordite, a substance essential for the British war industry during World War I.

Clostridium beijerinckii is a gram positive, rod shaped, motile bacterium of the genus Clostridium. It has been isolated from feces and soil. Produces oval to subterminal spores. it is named after Martinus Beijerinck who is a Dutch bacteriologist.
Thermoanaerobacter is a genus in the phylum Bacillota (Bacteria). Members of this genus are thermophilic and anaerobic, several of them were previously described as Clostridium species and members of the now obsolete genera Acetogenium and Thermobacteroides
Caldicellulosiruptor bescii is a species of thermophilic, anaerobic cellulolytic bacteria. It was isolated from a geothermally heated freshwater pool in the Valley of Geysers on the Kamchatka Peninsula in Russia in 1990. The species was originally named Anaerocellum thermophilum, but reclassified in 2010, based on genomic data.
Clostridium autoethanogenum is an anaerobic bacterium that produces ethanol from carbon monoxide, in so-called syngas fermentation, being one of the few known microorganisms to do so. It is gram-positive, spore-forming, rod-like, motile, and was first isolated from rabbit feces. Its type strain is strain JA1-1. Its genome has been sequenced and the genes required for utilising carbon monoxide as a sole carbon and energy source have been determined.
Thermoclostridium stercorarium is a cellulolytic thermophilic bacterium. It is anaerobic, spore-forming and saccharoclastic, with cells being rod-shaped and 0.7 to 0.8 by 2.7 to 7.7 µm in size. Its genome has been sequenced.
Clostridium paradoxum is a moderately thermophilic anaerobic alkaliphile bacteria. It is motile with 2-6 peritrichous flagella and forms round to slightly oval terminal spores. Its type strain is JW-YL-7.
Clostridium saccharoperbutylacetonicum is an indole and notably butanol-producing bacterium, with the type strain N1-4 (HMT). Its genome has been sequenced.
Acetivibrio straminisolvens is a moderately thermophilic, aerotolerant and cellulolytic bacterium. It is non-motile, spore-forming, straight or slightly curved rod, with type strain CSK1T. Its genome has been sequenced.
Clostridium pasteurianum is a bacterium discovered in 1890 by the Russian microbiologist Sergei Winogradsky. It was the first free living (non-symbiotic) micro-organism discovered that could fix free nitrogen from the air.
Pelobacter acetylenicus is a strictly anaerobic Gram-negative rod-shaped non-sporeforming bacterium of the genus Pelobacter. It was isolated from marine and freshwater sediments and can use acetylene (ethyne) as sole source of carbon and energy.
Clostridium carboxidivorans is a Gram-positive anaerobic, spore-forming and motile bacterium from the genus Clostridium which has been isolated from an agricultural lagoon in Oklahoma in the United States.
Clostridium hydrogeniformans is a Gram-positive, anaerobic, hydrogen-producing, spore-forming and motile bacterium from the genus Clostridium which has been isolated from groundwater in the United States.

Solventogenesis is the biochemical production of solvents by Clostridium species. It is the second phase of ABE fermentation.






