
Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some bioluminescent bacteria, and terrestrial arthropods such as fireflies. In some animals, the light is bacteriogenic, produced by symbiotic bacteria such as those from the genus Vibrio; in others, it is autogenic, produced by the animals themselves.

Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence, and is usually distinguished from a photoprotein. The name was first used by Raphaël Dubois who invented the words luciferin and luciferase, for the substrate and enzyme, respectively. Both words are derived from the Latin word lucifer, meaning "lightbearer", which in turn is derived from the Latin words for "light" (lux) and "to bring or carry" (ferre).

Luciferin is a generic term for the light-emitting compound found in organisms that generate bioluminescence. Luciferins typically undergo an enzyme-catalyzed reaction with molecular oxygen. The resulting transformation, which usually involves splitting off a molecular fragment, produces an excited state intermediate that emits light upon decaying to its ground state. The term may refer to molecules that are substrates for both luciferases and photoproteins.

Aequorin is a calcium-activated photoprotein isolated from the hydrozoan Aequorea victoria. Its bioluminescence was studied decades before the protein was isolated from the animal by Osamu Shimomura in 1962. In the animal, the protein occurs together with the green fluorescent protein to produce green light by resonant energy transfer, while aequorin by itself generates blue light.

Firefly luciferin is the luciferin, or light-emitting compound, used for the firefly (Lampyridae), railroad worm (Phengodidae), starworm (Rhagophthalmidae), and click-beetle (Pyrophorini) bioluminescent systems. It is the substrate of luciferase, which is responsible for the characteristic yellow light emission from many firefly species.
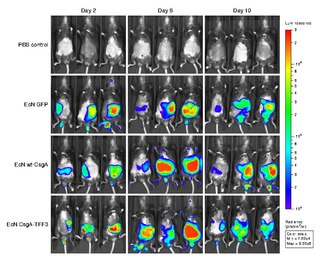
Bioluminescence imaging (BLI) is a technology developed over the past decades (1990's and onward). that allows for the noninvasive study of ongoing biological processes Recently, bioluminescence tomography (BLT) has become possible and several systems have become commercially available. In 2011, PerkinElmer acquired one of the most popular lines of optical imaging systems with bioluminescence from Caliper Life Sciences.

The slendertail lanternshark or Moller's lanternshark is a shark of the family Etmopteridae found in the western Indian Ocean between latitudes 34°N and 46°S at depths between 250 and 860 m. It can grow up to 46 cm in length.

The splendid lanternshark is a shark of the family Etmopteridae found in the western Pacific at depths between 120 and 210 m. Through the classification of Etmopterus species into several clades based on the positioning of their bioluminescent photophores, the splendid lanternshark can be considered a member of the Etmopterus pusillus clade.
In enzymology, a Cypridina-luciferin 2-monooxygenase (EC 1.13.12.6) is an enzyme that catalyzes the chemical reaction
In enzymology, an Oplophorus-luciferin 2-monooxygenase, also known as Oplophorus luciferase is a luciferase, an enzyme, from the deep-sea shrimp Oplophorus gracilirostris [2], belonging to a group of coelenterazine luciferases. Unlike other luciferases, it has a broader substrate specificity [3,4,6] and can also bind to bisdeoxycoelenterazine efficiently [3,4]. It is the third example of a luciferase to be purified in lab [2]. The systematic name of this enzyme class is Oplophorus-luciferin:oxygen 2-oxidoreductase (decarboxylating). This enzyme is also called Oplophorus luciferase.
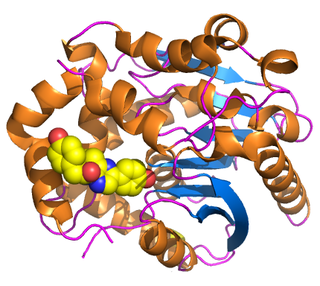
Renilla-luciferin 2-monooxygenase, Renilla luciferase, or RLuc, is a bioluminescent enzyme found in Renilla reniformis, belonging to a group of coelenterazine luciferases. Of this group of enzymes, the luciferase from Renilla reniformis has been the most extensively studied, and due to its bioluminescence requiring only molecular oxygen, has a wide range of applications, with uses as a reporter gene probe in cell culture, in vivo imaging, and various other areas of biological research. Recently, chimeras of RLuc have been developed and demonstrated to be the brightest luminescent proteins to date, and have proved effective in both noninvasive single-cell and whole body imaging.

Coelenterazine is a luciferin, a molecule that emits light after reaction with oxygen, found in many aquatic organisms across eight phyla. It is the substrate of many luciferases such as Renilla reniformis luciferase (Rluc), Gaussia luciferase (Gluc), and photoproteins, including aequorin, and obelin. All these proteins catalyze the oxidation of this substance, a reaction catalogued EC 1.13.12.5.

Vargulin, also called Cypridinid luciferin, Cypridina luciferin, or Vargula luciferin, is the luciferin found in the ostracod Cypridina hilgendorfii, also named Vargula hilgendorfii. These bottom dwelling ostracods emit a light stream into water when disturbed presumably to deter predation. Vargulin is also used by the midshipman fish, Porichthys.

Vargula hilgendorfii, sometimes called the sea-firefly and one of three bioluminescent species known in Japan as umi-hotaru (海蛍), is a species of ostracod crustacean. It is the only member of genus Vargula to inhabit Japanese waters; all other members of its genus inhabit the Gulf of Mexico, the Caribbean Sea, and waters off the coast of California. V. hilgendorfii was formerly more common, but its numbers have fallen significantly.

John Woodland "Woody" Hastings, was a leader in the field of photobiology, especially bioluminescence, and was one of the founders of the field of circadian biology. He was the Paul C. Mangelsdorf Professor of Natural Sciences and Professor of Molecular and Cellular Biology at Harvard University. He published over 400 papers and co-edited three books.

Bioluminescent bacteria are light-producing bacteria that are predominantly present in sea water, marine sediments, the surface of decomposing fish and in the gut of marine animals. While not as common, bacterial bioluminescence is also found in terrestrial and freshwater bacteria. These bacteria may be free living or in symbiosis with animals such as the Hawaiian Bobtail squid or terrestrial nematodes. The host organisms provide these bacteria a safe home and sufficient nutrition. In exchange, the hosts use the light produced by the bacteria for camouflage, prey and/or mate attraction. Bioluminescent bacteria have evolved symbiotic relationships with other organisms in which both participants benefit close to equally. Another possible reason bacteria use luminescence reaction is for quorum sensing, an ability to regulate gene expression in response to bacterial cell density.

Scintillons are small structures in cytoplasm that produce light. Among bioluminescent organisms, only dinoflagellates have scintillons.
Eukrohnia fowleri is a deep-sea marine arrow worm. It is the only known bioluminescent member of the genus Eukrohnia, and one of the two known species of bioluminescent arrow worms, the other being the distantly related Caecosagitta macrocephala. The bioluminescent organ of Eukrohnia fowleri is found along the center of its tail fin on both its dorsal and ventral side. It has a secreted bioluminescence that is thought to be coelenterazine based. While both species use luciferases in conjunction with coelenterazine for light emission, the luciferase of Eukrohnia fowleri is highly stable after 30 minutes while the luciferase of Caecosagitta macrocephala becomes inactive. So far, there is no other bioluminescent organism that uses hexagonal packing in order to hold bioluminescent materials/ E. fowleri evolved through the adaptation to hypoxic water and due to the recent oxygenation of water they have been experiencing bottleneck events. These events have been seen as one of the reasons that E. fowleri have such low biodiversity.
Halocypridae is a family of ostracods belonging to the order Halocyprida. This family contains bioluminescent members such as Conchoecia pseudodiscophora, which are believed to use coelenterazine and a coelenterazine luciferase rather than vargulin.














