Related Research Articles
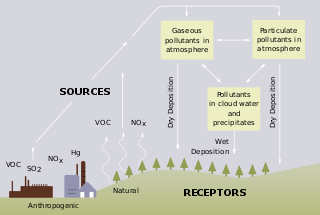
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions. Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average. The more acidic the acid rain is, the lower its pH is. Acid rain can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids.

An ecosystem consists of all the organisms and the physical environment with which they interact. These biotic and abiotic components are linked together through nutrient cycles and energy flows. Energy enters the system through photosynthesis and is incorporated into plant tissue. By feeding on plants and on one another, animals play an important role in the movement of matter and energy through the system. They also influence the quantity of plant and microbial biomass present. By breaking down dead organic matter, decomposers release carbon back to the atmosphere and facilitate nutrient cycling by converting nutrients stored in dead biomass back to a form that can be readily used by plants and microbes.

Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytoplankton productivity". Water bodies with very low nutrient levels are termed oligotrophic and those with moderate nutrient levels are termed mesotrophic. Advanced eutrophication may also be referred to as dystrophic and hypertrophic conditions. Eutrophication can affect freshwater or salt water systems. In freshwater ecosystems it is almost always caused by excess phosphorus. In coastal waters on the other hand, the main contributing nutrient is more likely to be nitrogen, or nitrogen and phosphorus together. This depends on the location and other factors.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Water pollution is the contamination of water bodies, usually as a result of human activities, so that it negatively affects its uses. Water bodies include lakes, rivers, oceans, aquifers, reservoirs and groundwater. Water pollution results when contaminants mix with these water bodies. Contaminants can come from one of four main sources: sewage discharges, industrial activities, agricultural activities, and urban runoff including stormwater. Water pollution is either surface water pollution or groundwater pollution. This form of pollution can lead to many problems, such as the degradation of aquatic ecosystems or spreading water-borne diseases when people use polluted water for drinking or irrigation. Another problem is that water pollution reduces the ecosystem services that the water resource would otherwise provide.

An air quality index (AQI) is used by government agencies to communicate to the public how polluted the air currently is or how polluted it is forecast to become. AQI information is obtained by averaging readings from an air quality sensor, which can increase due to vehicle traffic, forest fires, or anything that can increase air pollution. Pollutants tested include ozone, nitrogen dioxide, sulphur dioxide, among others.

Marine pollution occurs when substances used or spread by humans, such as industrial, agricultural and residential waste, particles, noise, excess carbon dioxide or invasive organisms enter the ocean and cause harmful effects there. The majority of this waste (80%) comes from land-based activity, although marine transportation significantly contributes as well. Since most inputs come from land, either via the rivers, sewage or the atmosphere, it means that continental shelves are more vulnerable to pollution. Air pollution is also a contributing factor by carrying off iron, carbonic acid, nitrogen, silicon, sulfur, pesticides or dust particles into the ocean. The pollution often comes from nonpoint sources such as agricultural runoff, wind-blown debris, and dust. These nonpoint sources are largely due to runoff that enters the ocean through rivers, but wind-blown debris and dust can also play a role, as these pollutants can settle into waterways and oceans. Pathways of pollution include direct discharge, land runoff, ship pollution, atmospheric pollution and, potentially, deep sea mining.

Nonpoint source (NPS) pollution refers to diffuse contamination of water or air that does not originate from a single discrete source. This type of pollution is often the cumulative effect of small amounts of contaminants gathered from a large area. It is in contrast to point source pollution which results from a single source. Nonpoint source pollution generally results from land runoff, precipitation, atmospheric deposition, drainage, seepage, or hydrological modification where tracing pollution back to a single source is difficult. Nonpoint source water pollution affects a water body from sources such as polluted runoff from agricultural areas draining into a river, or wind-borne debris blowing out to sea. Nonpoint source air pollution affects air quality, from sources such as smokestacks or car tailpipes. Although these pollutants have originated from a point source, the long-range transport ability and multiple sources of the pollutant make it a nonpoint source of pollution; if the discharges were to occur to a body of water or into the atmosphere at a single location, the pollution would be single-point.

Surface runoff is the unconfined flow of water over the ground surface, in contrast to channel runoff. It occurs when excess rainwater, stormwater, meltwater, or other sources, can no longer sufficiently rapidly infiltrate in the soil. This can occur when the soil is saturated by water to its full capacity, and the rain arrives more quickly than the soil can absorb it. Surface runoff often occurs because impervious areas do not allow water to soak into the ground. Furthermore, runoff can occur either through natural or man-made processes. Surface runoff is a major component of the water cycle. It is the primary agent of soil erosion by water. The land area producing runoff that drains to a common point is called a drainage basin.
Soil acidification is the buildup of hydrogen cations, which reduces the soil pH. Chemically, this happens when a proton donor gets added to the soil. The donor can be an acid, such as nitric acid, sulfuric acid, or carbonic acid. It can also be a compound such as aluminium sulfate, which reacts in the soil to release protons. Acidification also occurs when base cations such as calcium, magnesium, potassium and sodium are leached from the soil.

The Air Pollution Index is a simple and generalized way to describe the air quality, which is used in Malaysia. It is calculated from several sets of air pollution data and was formerly used in mainland China and Hong Kong. In mainland China the API was replaced by an updated air quality index in early 2012 and on 30 December 2013 Hong Kong moved to a health based index.

Human impact on the nitrogen cycle is diverse. Agricultural and industrial nitrogen (N) inputs to the environment currently exceed inputs from natural N fixation. As a consequence of anthropogenic inputs, the global nitrogen cycle (Fig. 1) has been significantly altered over the past century. Global atmospheric nitrous oxide (N2O) mole fractions have increased from a pre-industrial value of ~270 nmol/mol to ~319 nmol/mol in 2005. Human activities account for over one-third of N2O emissions, most of which are due to the agricultural sector. This article is intended to give a brief review of the history of anthropogenic N inputs, and reported impacts of nitrogen inputs on selected terrestrial and aquatic ecosystems.

A wild fishery is a natural body of water with a sizeable free-ranging fish or other aquatic animal population that can be harvested for its commercial value. Wild fisheries can be marine (saltwater) or lacustrine/riverine (freshwater), and rely heavily on the carrying capacity of the local aquatic ecosystem.
The 1999 Gothenburg Protocol to Abate Acidification, Eutrophication and Ground-level Ozone is a multi-pollutant protocol designed to reduce acidification, eutrophication and ground-level ozone by setting emissions ceilings for sulphur dioxide, nitrogen oxides, volatile organic compounds and ammonia to be met by 2010. As of August 2014, the Protocol had been ratified by 26 parties, which includes 25 states and the European Union.

Agricultural pollution refers to biotic and abiotic byproducts of farming practices that result in contamination or degradation of the environment and surrounding ecosystems, and/or cause injury to humans and their economic interests. The pollution may come from a variety of sources, ranging from point source water pollution to more diffuse, landscape-level causes, also known as non-point source pollution and air pollution. Once in the environment these pollutants can have both direct effects in surrounding ecosystems, i.e. killing local wildlife or contaminating drinking water, and downstream effects such as dead zones caused by agricultural runoff is concentrated in large water bodies.

Nutrient pollution, a form of water pollution, refers to contamination by excessive inputs of nutrients. It is a primary cause of eutrophication of surface waters, in which excess nutrients, usually nitrogen or phosphorus, stimulate algal growth. Sources of nutrient pollution include surface runoff from farm fields and pastures, discharges from septic tanks and feedlots, and emissions from combustion. Raw sewage is a large contributor to cultural eutrophication since sewage is high in nutrients. Releasing raw sewage into a large water body is referred to as sewage dumping, and still occurs all over the world. Excess reactive nitrogen compounds in the environment are associated with many large-scale environmental concerns. These include eutrophication of surface waters, harmful algal blooms, hypoxia, acid rain, nitrogen saturation in forests, and climate change.

Pollution is an environmental issue in Canada. It has posed health risks to the Canadian population and is an area of concern for Canadian lawmakers. Air, water and soil pollution as well as the associated health effects are prominent points of contention in modern Canadian society.

Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas, or by the reduction of acid anions, like sulfate and nitrate within a lake. Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching. Carbonic acid and dissolved carbon dioxide can also enter freshwaters in a similar manner associated with runoff through carbon dioxide-rich soils. Runoff that contains these compounds may incorporate acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds.

Biodiversity loss includes the worldwide extinction of different species, as well as the local reduction or loss of species in a certain habitat, resulting in a loss of biological diversity. The latter phenomenon can be temporary or permanent, depending on whether the environmental degradation that leads to the loss is reversible through ecological restoration/ecological resilience or effectively permanent. The current global extinction, has resulted in a biodiversity crisis being driven by human activities which push beyond the planetary boundaries and so far has proven irreversible.

Human activities affect marine life and marine habitats through overfishing, habitat loss, the introduction of invasive species, ocean pollution, ocean acidification and ocean warming. These impact marine ecosystems and food webs and may result in consequences as yet unrecognised for the biodiversity and continuation of marine life forms.
References
- ↑ Nilsson, J. and P. Grennfelt. 1988. Critical loads for sulphur and nitrogen. UNECE/Nordic Council workshop report, Skokloster, Sweden. March 1988.
- 1 2 Greaver, T. L., T. J. Sullivan, J. D. Herrick, M. C. Barber, J. S. Baron, B. J. Cosby, M. E. Deerhake, R. L. Dennis, J.-J. B. Dubois, C. L. Goodale, A. T. Herlihy, G. B. Lawrence, L. Liu, J. A. Lynch, and K. J. Novak. 2012. Ecological effects of nitrogen and sulfur air pollution in the US: what do we know? Frontiers in Ecology and the Environment 10:365-372.
- ↑ Driscoll, C. T., G. B. Lawrence, A. J. Bulger, T. J. Butler, C. S. Cronan, C. Eagar, K. F. Lambert, G. E. Likens, J. L. Stoddard, and K. C. Weathers. 2001. Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies. BioScience 51:180-198.
- 1 2 Bobbink, R., K. Hicks, J. Galloway, T. Spranger, R. Alkemade, M. Ashmore, M. Bustamante, S. Cinderby, E. Davidson, F. Dentener, B. Emmett, J.-W. Erisman, M. Fenn, F. Gilliam, A. Nordin, L. Pardo, and W. De Vries. 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications 20:30-59.
- 1 2 Pardo, L. H., M. E. Fenn, C. L. Goodale, L. H. Geiser, C. T. Driscoll, E. B. Allen, J. S. Baron, R. Bobbink, W. D. Bowman, C. M. Clark, B. Emmett, F. S. Gilliam, T. L. Greaver, S. J. Hall, E. A. Lilleskov, L. Liu, J. A. Lynch, K. J. Nadelhoffer, S. S. Perakis, M. J. Robin-Abbott, J. L. Stoddard, K. C. Weathers, and R. L. Dennis. 2011. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecological Applications 21:3049-3082.
- 1 2 3 Liu, X.J., L. Duan, J.M. Mo, E.Z. Du, J.L. Shen, X.K. Lu, Y. Zhang, X.B. Zhou, C.E. He, and F.S. Zhang. 2011. Nitrogen deposition and its ecological impact in China: an overview. Environmental Pollution 159:2251-2264.
- 1 2 Duan, L., Q. Yu, Q. Zhang, Z. Wang, Y. Pan, T. Larssen, J. Tang, and J. Mulder. 2016. Acid deposition in Asia: emissions, deposition, and ecosystem effects. Atmospheric Environment 146:55-69.
- ↑ Hettelingh, J.P., H. Sverdrup, and D. Zhao. 1995. Deriving critical loads for Asia. Water, Air, and Soil Pollution 85(4):2565-2570.