
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.

Percy Lavon Julian was an American research chemist and a pioneer in the chemical synthesis of medicinal drugs from plants. He was the first to synthesize the natural product physostigmine and was a pioneer in the industrial large-scale chemical synthesis of the human hormones progesterone and testosterone from plant sterols such as stigmasterol and sitosterol. His work laid the foundation for the steroid drug industry's production of cortisone, other corticosteroids, and birth control pills.

Prednisone is a glucocorticoid medication mostly used to suppress the immune system and decrease inflammation in conditions such as asthma, COPD, and rheumatologic diseases. It is also used to treat high blood calcium due to cancer and adrenal insufficiency along with other steroids. It is taken by mouth.

Hydrocortisone is the name for the hormone cortisol when supplied as a medication. Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD. It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly.

A rash is a change of the human skin that affects its color, appearance, or texture.

Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.
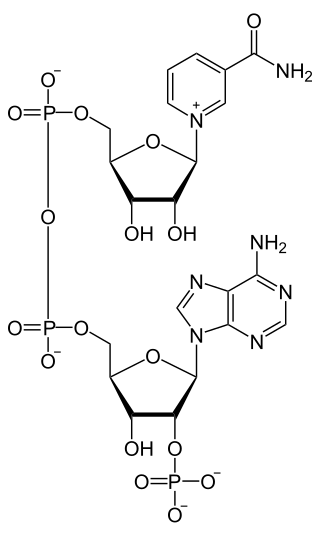
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.

Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH3(CH2)3COOH. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. It is found in the perennial flowering plant Valeriana officinalis, from which it gets its name. Its primary use is in the synthesis of its esters. Salts and esters of valeric acid are known as valerates or pentanoates. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Several, including ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.
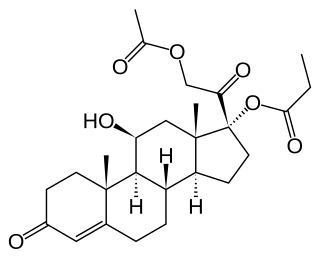
Hydrocortisone aceponate is a veterinary corticosteroid that is used in form of creams for the treatment of various dermatoses. It is an ester of hydrocortisone (cortisol) with acetic acid and propionic acid.
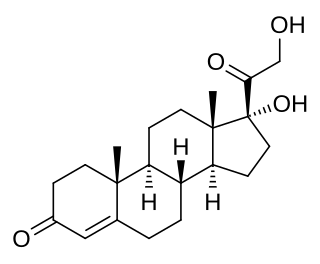
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate towards cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.
Critical illness–related corticosteroid insufficiency is a form of adrenal insufficiency in critically ill patients who have blood corticosteroid levels which are inadequate for the severe stress response they experience. Combined with decreased glucocorticoid receptor sensitivity and tissue response to corticosteroids, this adrenal insufficiency constitutes a negative prognostic factor for intensive care patients.
Topical steroids are the topical forms of corticosteroids. Topical steroids are the most commonly prescribed topical medications for the treatment of rash and eczema. Topical steroids have anti-inflammatory properties and are classified based on their skin vasoconstrictive abilities. There are numerous topical steroid products. All the preparations in each class have the same anti-inflammatory properties but essentially differ in base and price.
Cunninghamella elegans is a species of fungus in the genus Cunninghamella found in soil.

The class Zetaproteobacteria is the sixth and most recently described class of the Pseudomonadota. Zetaproteobacteria can also refer to the group of organisms assigned to this class. The Zetaproteobacteria were originally represented by a single described species, Mariprofundus ferrooxydans, which is an iron-oxidizing neutrophilic chemolithoautotroph originally isolated from Kamaʻehuakanaloa Seamount in 1996 (post-eruption). Molecular cloning techniques focusing on the small subunit ribosomal RNA gene have also been used to identify a more diverse majority of the Zetaproteobacteria that have as yet been unculturable.

Epiestriol, or epioestriol, also known as 16β-epiestriol or simply 16-epiestriol as well as 16β-hydroxy-17β-estradiol, is a minor and weak endogenous estrogen, and the 16β-epimer of estriol. Epiestriol is used clinically in the treatment of acne. In addition to its estrogenic actions, epiestriol has been found to possess significant anti-inflammatory properties without glycogenic activity or immunosuppressive effects, an interesting finding that is in contrast to conventional anti-inflammatory steroids like hydrocortisone.

Cunninghamella echinulata is a fungal species in the genus Cunninghamella. It is an asexually reproducing fungus and a mesophile, preferring intermediate temperature ranges. C. echinulata is a common air contaminant, and is currently of interest to the biotechnology industry due to its ability to synthesize γ-linolenic acid as well as its capacity to bioconcentrate metals. This species is a soil saprotroph that forms rhizoids, preferring soils enriched in nitrogen, phosphorus and potassium. It has been reported occasionally an agent of mucormycosis following the inhalation of fungal spores. Czapek's agar is a suitable growth medium for the propagation of C. echinulata.
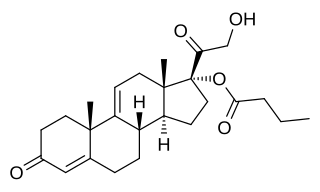
9,11-Dehydrocortexolone 17α-butyrate is a synthetic steroidal antiandrogen that was developed for use as a topical medication but was never marketed. It is the C17α butyrate (butanoate) ester of the 9,11-dehydrogenated analogue of 11-deoxycortisol (cortexolone). C17α esters of 11-deoxycortisol were unexpectedly found to possess antiandrogen activity, and 9,11-dehydrocortexolone 17α-butyrate was selected for development based on its optimum drug profile. In rats, the drug has been found to possess strong local antiandrogen activity and to also be active systemically upon subcutaneous injection. In addition, it has been found to possess antigonadotropic activity. In terms of topical antiandrogen potency, 9,11-dehydrocortexolone 17α-butyrate was found to be more potent than flutamide and finasteride and less than or equal to cyproterone acetate in potency. The drug has been suggested for possible development and medical use for the treatment of prostate cancer and benign prostatic hyperplasia.

Domoprednate is a synthetic glucocorticoid corticosteroid which was developed in the late 1970s and 1980s.














