
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses are as treatments for congestive heart failure and cardiac arrhythmias; however, their relative toxicity prevents them from being widely used. Most commonly found as secondary metabolites in several plants such as foxglove plants, these compounds nevertheless have a diverse range of biochemical effects regarding cardiac cell function and have also been suggested for use in cancer treatment.

The Crassulaceae, also known as the stonecrop family or the orpine family, are a diverse family of dicotyledon flowering plants characterized by succulent leaves and a unique form of photosynthesis, known as Crassulacean acid metabolism (CAM). Flowers generally have five floral parts. Crassulaceae are usually herbaceous but there are some subshrubs, and relatively few treelike or aquatic plants. Crassulaceae are a medium size monophyletic family in the core eudicots, among the order Saxifragales, whose diversity has made infrafamilial classification very difficult. The family includes approximately 1,400 species and 34–35 genera, depending on the circumscription of the genus Sedum, and distributed over three subfamilies. Members of the Crassulaceae are found worldwide, but mostly in the Northern Hemisphere and southern Africa, typically in dry and/or cold areas where water may be scarce, although a few are aquatic.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.

KalanchoeKAL-ən-KOH-ee, also written Kalanchöe or Kalanchoë, is a genus of about 125 species of tropical, succulent plants in the stonecrop family Crassulaceae, mainly native to Madagascar and tropical Africa. A Kalanchoe species was one of the first plants to be sent into space, sent on a resupply to the Soviet Salyut 1 space station in 1979. The majority of kalanchoes require around 6-8 hours of sunlight a day; a few cannot tolerate this, and survive with bright, indirect sunlight to bright shade.

Ouabain or also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an arrow poison in eastern Africa for both hunting and warfare. Ouabain is a cardiac glycoside and in lower doses, can be used medically to treat hypotension and some arrhythmias. It acts by inhibiting the Na/K-ATPase, also known as the sodium-potassium ion pump. However, adaptations to the alpha-subunit of the Na+/K+-ATPase via amino acid substitutions, have been observed in certain species, namely some herbivore- insect species, that have resulted in toxin resistance.
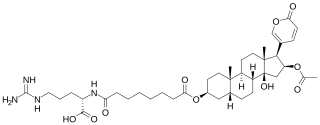
Bufotoxins are a family of toxic steroid lactones or substituted tryptamines of which some are toxic. They occur in the parotoid glands, skin, and poison of many toads and other amphibians, and in some plants and mushrooms. The exact composition varies greatly with the specific source of the toxin. It can contain 5-MeO-DMT, bufagins, bufalin, bufotalin, bufotenin, bufothionine, dehydrobufotenine, epinephrine, norepinephrine, and serotonin. Some authors have also used the term bufotoxin to describe the conjugate of a bufagin with suberylarginine.

Kalanchoe beharensis is a plant species in the succulent genus Kalanchoe, and the family Crassulaceae. Kalanchoe beharensis is native to Madagascar known by local names mongy, rongy and tavitavy.

Bryophyllum is a group of plant species of the family Crassulaceae native to Madagascar. It is a section or subgenus within the genus Kalanchoe, and was formerly placed at the level of genus. This section is notable for vegetatively growing small plantlets on the fringes of the leaves; these eventually drop off and root. These plantlets arise from mitosis of meristematic-type tissue in notches in the leaves.

Kalanchoe daigremontiana, formerly known as Bryophyllum daigremontianum and commonly called mother of thousands, or Mexican hat plant, is a succulent plant native to Madagascar. Like other members of Bryophyllum, it can propagate vegetatively from plantlets that develop on its leaf margins, as well as through upshoots from lateral roots, and seeds. All parts of this species contain a very toxic steroid known as daigremontianin.
Arrow poisons are used to poison arrow heads or darts for the purposes of hunting and warfare. They have been used by indigenous peoples worldwide and are still in use in areas of South America, Africa and Asia. Notable examples are the poisons secreted from the skin of the poison dart frog, and curare, a general term for a range of plant-derived arrow poisons used by the indigenous peoples of South America.

A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting. Cardenolides are toxic to animals through inhibition of the enzyme Na+/K+‐ATPase, which is responsible for maintaining the sodium and potassium ion gradients across the cell membranes.
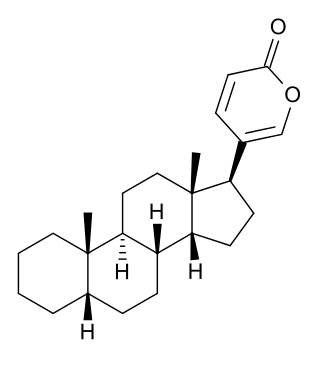
Bufadienolide is a chemical compound with steroid structure. Its derivatives are collectively known as bufadienolides, including many in the form of bufadienolide glycosides. These are a type of cardiac glycoside, the other being the cardenolide glycosides. Both bufadienolides and their glycosides are toxic; specifically, they can cause an atrioventricular block, bradycardia, ventricular tachycardia, and possibly lethal cardiac arrest.

k-Strophanthidin is a cardenolide found in species of the genus Strophanthus. It is the aglycone of k-strophanthin, an analogue of ouabain. k-strophanthin is found in the ripe seeds of Strophanthus kombé and in the lily Convallaria.

Drimia maritima is a species of flowering plant in the family Asparagaceae, subfamily Scilloideae. This species is known by several common names, including squill, sea squill, sea onion, and maritime squill. It may also be called red squill, particularly a form which produces red-tinged flowers instead of white. It is native to southern Europe, western Asia, and northern Africa.

Kalanchoe delagoensis, formerly known as Bryophyllum delagoense and commonly called mother of millions or chandelier plant, is a succulent plant native to Madagascar. Like other members of Bryophyllum, it is able to propagate vegetatively from plantlets that develop on its leaf margins.

Kalanchoe pinnata, commonly known as cathedral bells, air plant, life plant, miracle leaf, and Goethe plant is a succulent plant native to Madagascar. It is a popular houseplant and has become naturalized in tropical and subtropical areas. The species is distinctive for the profusion of miniature plantlets that form on the margins of its leaves, a trait it has in common with some other members of Bryophyllum.

Cotyledon tomentosa is a species of flowering plant in the family Crassulaceae, native to South Africa. It is a succulent evergreen shrub with large chunky ovate fuzzy green leaves. Its autonymous subspecies is known as the bear's paw because of the prominent "teeth" at the tips of its leaves. It forms large orange bell-shaped flowers in spring. In its native habitat, the Little Karoo region of South Africa, Cotyledons usually grow in rocky quartz fields where they have excellent drainage provided by very porous soil.

Arenobufagin is a cardiotoxic bufanolide steroid secreted by the Argentine toad Bufo arenarum. It has effects similar to digitalis, blocking the Na+/K+ pump in heart tissue.

Tylecodon wallichii is a species of succulent plant in the genus Tylecodon belonging to the family Crassulaceae. The species is named in honour of Nathaniel Wallich, early 19th century Danish plant hunter, botanist and physician.

Tylecodon paniculatus, also known as butter bush, butter tree, butterboom or rooisuikerblom (Afrikaans), is a species of succulent plant in the genus Tylecodon belonging to the family Crassulaceae.



















