Related Research Articles

The insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin. IGFs are part of a complex system that cells use to communicate with their physiologic environment. This complex system consists of two cell-surface receptors, two ligands, a family of seven high-affinity IGF-binding proteins, as well as associated IGFBP degrading enzymes, referred to collectively as proteases.

Parasitism is a close relationship between species, where one organism, the parasite, lives on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The entomologist E. O. Wilson characterised parasites as "predators that eat prey in units of less than one". Parasites include single-celled protozoans such as the agents of malaria, sleeping sickness, and amoebic dysentery; animals such as hookworms, lice, mosquitoes, and vampire bats; fungi such as honey fungus and the agents of ringworm; and plants such as mistletoe, dodder, and the broomrapes.

Caenorhabditis elegans is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a blend of the Greek caeno- (recent), rhabditis (rod-like) and Latin elegans (elegant). In 1900, Maupas initially named it Rhabditides elegans. Osche placed it in the subgenus Caenorhabditis in 1952, and in 1955, Dougherty raised Caenorhabditis to the status of genus.

In biology and medicine, a host is a larger organism that harbours a smaller organism; whether a parasitic, a mutualistic, or a commensalist guest (symbiont). The guest is typically provided with nourishment and shelter. Examples include animals playing host to parasitic worms, cells harbouring pathogenic (disease-causing) viruses, or a bean plant hosting mutualistic (helpful) nitrogen-fixing bacteria. More specifically in botany, a host plant supplies food resources to micropredators, which have an evolutionarily stable relationship with their hosts similar to ectoparasitism. The host range is the collection of hosts that an organism can use as a partner.
The DAF-2 gene encodes for the insulin-like growth factor 1 (IGF-1) receptor in the worm Caenorhabditis elegans. DAF-2 is part of the first metabolic pathway discovered to regulate the rate of aging. DAF-2 is also known to regulate reproductive development, resistance to oxidative stress, thermotolerance, resistance to hypoxia, and resistance to bacterial pathogens. Mutations in DAF-2 and also Age-1 have been shown by Cynthia Kenyon to double the lifespan of the worms. In a 2007 episode of WNYC’s Radiolab, Kenyon called DAF-2 "the grim reaper gene.”

Root-knot nematodes are plant-parasitic nematodes from the genus Meloidogyne. They exist in soil in areas with hot climates or short winters. About 2000 plants worldwide are susceptible to infection by root-knot nematodes and they cause approximately 5% of global crop loss. Root-knot nematode larvae infect plant roots, causing the development of root-knot galls that drain the plant's photosynthate and nutrients. Infection of young plants may be lethal, while infection of mature plants causes decreased yield.

A polyphenic trait is a trait for which multiple, discrete phenotypes can arise from a single genotype as a result of differing environmental conditions. It is therefore a special case of phenotypic plasticity.

Caenorhabditis is a genus of nematodes which live in bacteria-rich environments like compost piles, decaying dead animals and rotting fruit. The name comes from Greek: caeno- ; rhabditis = rod-like.

The alae is a protruding ridge that forms longitudinally on many nematodes. In the Caenorhabditis elegans nematode they are present in the L1, dauer and adult stages. The alae are most pronounced during the dauer larval stage and not present in the L2, and L3 C. elegans stages.

The nematodes, roundworms or eelworms constitute the phylum Nematoda. They are a diverse animal phylum inhabiting a broad range of environments. Most species are free-living, feeding on microorganisms, but many species are parasitic. The parasitic worms (helminths) are the cause of soil-transmitted helminthiases.
DAF-16 is the sole ortholog of the FOXO family of transcription factors in the nematode Caenorhabditis elegans. It is responsible for activating genes involved in longevity, lipogenesis, heat shock survival and oxidative stress responses. It also protects C.elegans during food deprivation, causing it to transform into a hibernation - like state, known as a Dauer. DAF-16 is notable for being the primary transcription factor required for the profound lifespan extension observed upon mutation of the insulin-like receptor DAF-2. The gene has played a large role in research into longevity and the insulin signalling pathway as it is located in C. elegans, a successful ageing model organism.

Phoresis or phoresy is a temporary commensalistic relationship when an organism attaches itself to a host organism solely for travel. It has been seen in ticks and mites since the 18th century, and in fossils 320 million years old. It is not restricted to arthropods or animals; plants with seeds that disperse by attaching themselves to animals are also considered to be phoretic.
Caenorhabditis japonica is a species of nematodes in the genus Caenorhabditis. Its genome was sequenced by the McDonnell Genome Institute at Washington University School of Medicine. This gonochoristic species is found in the 'Japonica' group, the sister clade to the 'Elegans' group, in the 'Elegans' supergroup.
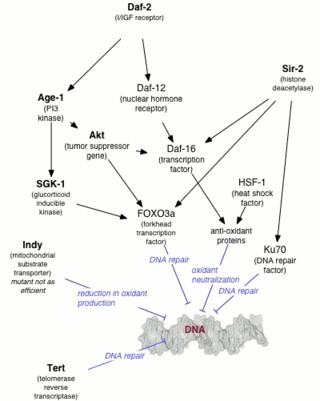
Genetics of aging is generally concerned with life extension associated with genetic alterations, rather than with accelerated aging diseases leading to reduction in lifespan.

Pristionchus pacificus is a species of free-living nematodes (roundworms) in the family Diplogastridae. The species has been established as a satellite model organism to Caenorhabditis elegans, with which it shared a common ancestor 200–300 million years ago. The genome of P. pacificus has been fully sequenced, which in combination with other tools for genetic analysis make this species a tractable model in the laboratory, especially for studies of developmental biology.

Caenorhabditis elegans- microbe interactions are defined as any interaction that encompasses the association with microbes that temporarily or permanently live in or on the nematode C. elegans. The microbes can engage in a commensal, mutualistic or pathogenic interaction with the host. These include bacterial, viral, unicellular eukaryotic, and fungal interactions. In nature C. elegans harbours a diverse set of microbes. In contrast, C. elegans strains that are cultivated in laboratories for research purposes have lost the natural associated microbial communities and are commonly maintained on a single bacterial strain, Escherichia coli OP50. However, E. coli OP50 does not allow for reverse genetic screens because RNAi libraries have only been generated in strain HT115. This limits the ability to study bacterial effects on host phenotypes. The host microbe interactions of C. elegans are closely studied because of their orthologs in humans. Therefore, the better we understand the host interactions of C. elegans the better we can understand the host interactions within the human body.
Worm bagging is a form of vivipary observed in nematodes, namely Caenorhabditis elegans. The process is characterized by eggs hatching within the parent and the larvae proceeding to consume and emerge from the parent.
The DAF-12 gene encodes the nuclear receptor of dafachronic acid in the worm Caenorhabditis elegans, with the NRNC Symbol NR1J1 as the homolog of nuclear hormone receptor HR96 in Drosophila melanogaster. DAF-12 has been implicated by Cynthia Kenyon and colleagues in the formation of Dauer larva.
The Daf-9 gene encodes a cytochrome p450 enzyme catalysis the generation of dafachronic acid in the worm Caenorhabditis elegans, with the CYP Symbol CYP22A1. After generation, dafachronic acid will binding it's nuclear receptor Daf-12 and has been implicated by Cynthia Kenyon and colleagues related to the formation of Dauer larva.
Daf-5 is an ortholog of the mammalian protein Sno/Ski,which present in the nematode worm Caenorhabditis elegans on the downstream of TGFβ signaling pathway. Without daf-7 signal, daf-5 combined with daf-3, co-SMAD for C. elegans, to form a heterodimer and started dauer development.
References
- ↑ Fuchs, Anton Gilbert (1937). Neue parasitische und halbparasitische Nematoden bei Borkenkäfern und einige andere Nematoden[New Parasitic and Half-parasitic Nematodes with Bark-Beetles and Some Other Nematodes] (in German). Fischer.
- 1 2 3 4 Roy C. Anderson (8 February 2000). Nematode Parasites of Vertebrates: Their Development and Transmission. CABI. pp. 4–5. ISBN 978-0-85199-786-5.
- ↑ Riddle DL, Swanson MM, Albert PS (1981). "Interacting genes in nematode dauer larva formation". Nature . 290 (5808): 668–671. Bibcode:1981Natur.290..668R. doi:10.1038/290668a0. PMID 7219552. S2CID 4255657.
- ↑ Hu, Patrick J. (2007). "Dauer". WormBook: 1–19. doi:10.1895/wormbook.1.144.1. ISSN 1551-8507. PMC 2890228 . PMID 17988074 . Retrieved 2009-11-05.
- ↑ Sommer, Ralf J.; Akira Ogawa (September 2011). "Hormone Signaling and Phenotypic Plasticity in Nematode Development and Evolution". Current Biology. 21 (18): R758–R766. Bibcode:2011CBio...21.R758S. doi: 10.1016/j.cub.2011.06.034 . ISSN 0960-9822. PMID 21959166.
- ↑ RIDDLE, D.. 12 The Dauer Larva. Cold Spring Harbor Monograph Archive, North America, 17 January 1988. Available at: https://cshmonographs.org/index.php/monographs/article/view/5027/4126. Date accessed: 14 July 2016.
- ↑ Mayer, Melanie G.; Ralf J. Sommer (2011). "Natural variation in Pristionchus pacificus dauer formation reveals cross-preference rather than self-preference of nematode dauer pheromones". Proceedings of the Royal Society B: Biological Sciences. 278 (1719): 2784–2790. doi:10.1098/rspb.2010.2760. PMC 3145190 . PMID 21307052.
- ↑ Gottlieb S, Ruvkun G (1994). "daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans". Genetics . 137 (1): 107–120. doi:10.1093/genetics/137.1.107. PMC 1205929 . PMID 8056303.
- ↑ Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993). "A C. elegans mutant that lives twice as long as wild type". Nature . 366 (6454): 461–464. Bibcode:1993Natur.366..461K. doi:10.1038/366461a0. PMID 8247153. S2CID 4332206.
- ↑ Lithgow GJ, White TM, Melov S, Johnson TE (1995). "Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress". Proceedings of the National Academy of Sciences of the United States of America . 92 (16): 7540–7544. Bibcode:1995PNAS...92.7540L. doi: 10.1073/pnas.92.16.7540 . PMC 41375 . PMID 7638227.
- 1 2 Wolkow, C.A.; Hall, D.H. (2011). Herndon, Laura A. (ed.). "The Dauer Cuticle". WormAtlas. doi:10.3908/wormatlas.3.1 . Retrieved 6 September 2024.
- ↑ Galles, Celina; Prez, Gastón M.; Penkov, Sider; Boland, Sebastian; Porta, Exequiel O. J.; Altabe, Silvia G.; Labadie, Guillermo R.; Schmidt, Ulrike; Knölker, Hans-Joachim (2018-04-23). "Endocannabinoids in Caenorhabditis elegans are essential for the mobilization of cholesterol from internal reserves". Scientific Reports. 8 (1): 6398. Bibcode:2018NatSR...8.6398G. doi:10.1038/s41598-018-24925-8. ISSN 2045-2322. PMC 5913221 . PMID 29686301.
- ↑ Viney, Mark (June 2017). "How Can We Understand the Genomic Basis of Nematode Parasitism?". Trends in Parasitology. 33 (6): 444–452. doi: 10.1016/j.pt.2017.01.014 . PMC 5449551 . PMID 28274802.
- 1 2 Poniar Jr., G.O. (Jan 2018). Taxonomy and biology of Steinernematidae and Heterorhabditidae. CRC Press. pp. 23–58. ISBN 9781351088640 . Retrieved 6 December 2023.
- ↑ Félix, MA (2010). "The natural history of Caenorhabditis elegans". Current Biology. 20 (22): R965-9. Bibcode:2010CBio...20.R965F. doi: 10.1016/j.cub.2010.09.050 . PMID 21093785. S2CID 12869939 . Retrieved 6 December 2023.
- ↑ Kiontke, K. "Nematodes". Current Biology. Retrieved 6 December 2023.
- 1 2 Crook, Matt (2014). "The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong". International Journal for Parasitology. 44 (1): 1–8. doi:10.1016/j.ijpara.2013.08.004. PMC 3947200 . PMID 24095839.
- 1 2 3 Bubrig, Louis (2020). "Caenorhabditis elegans dauers vary recovery in response to bacteria from natural habitat". Ecology and Evolution. 10 (18). Ecology and Evolution Vol. 10: 9886–9895. Bibcode:2020EcoEv..10.9886B. doi:10.1002/ece3.6646. PMC 7520223 . PMID 33005351.
- ↑ Rebecchi, Lorena (2020). "Extreme-tolerance mechanisms in meiofaunal organisms: a case study with tardigrades, rotifers and nematodes". Hydrobiologia. 847 (12): 2779–2799. doi:10.1007/s10750-019-04144-6. hdl: 11380/1204602 . S2CID 209380774.
- ↑ Poulin, Robert (2015). "Evolution of parasitism along convergent lines: from ecology to genomics". Parasitology. 142 (Suppl 1). Cambridge University Press: S6–S15. doi:10.1017/S0031182013001674. PMC 4413784 . PMID 24229807.
- ↑ Anderson, R.C. (1984). "The origins of zooparasitic nematodes". Canadian Journal of Zoology. 62 (3): 317–328. doi:10.1139/z84-050.
- ↑ Houck, M.A. (1991). "Ecological and Evolutionary Significance of Phoresy in the Astigmata". Annual Review of Entomology. 36. Annual Reviews: 611–636. doi:10.1146/annurev.en.36.010191.003143 . Retrieved 6 December 2023.
- ↑ Heip, C.H.R (1985). The ecology of marine nematodes. Oceanography and Marine Biology: An Annual Review. pp. 399–489. Retrieved 6 December 2023.
- ↑ Ludewig, Andreas (2019). "An excreted small molecule promotes C. elegans reproductive development and aging". Nature Chemical Biology. 15 (8): 838–845. doi:10.1038/s41589-019-0321-7. PMC 6650165 . PMID 31320757.
- ↑ Blaxter, Max (2015). "The evolution of parasitism in Nematoda". Parasitology. 142 (Suppl 1). Cambridge University Press: S26–S39. doi:10.1017/S0031182014000791. PMC 4413787 . PMID 24963797.