
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.

In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
Protease inhibitors (PIs) are a class of antiviral drugs that are widely used to treat HIV/AIDS and hepatitis C. Protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

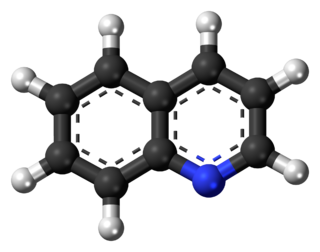
Isoquinoline is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler. Traditionally an acidic catalyst in protic solvent was employed with heating, however the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. This rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.
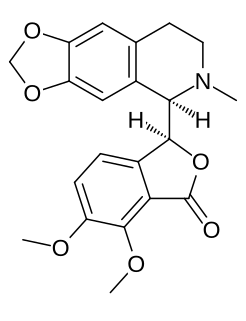
Hydrastine is an isoquinoline alkaloid which was discovered in 1851 by Alfred P. Durand. Hydrolysis of hydrastine yields hydrastinine, which was patented by Bayer as a haemostatic drug during the 1910s. It is present in Hydrastis canadensis and other plants of the family Ranunculaceae.

Isoindole in heterocyclic chemistry is a benzo-fused pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical literature, but substituted derivatives are useful commercially and occur naturally. Isoindoles units occur in phthalocyanines, an important family of dyes. Some alkaloids containing isoindole have been isolated and characterized.

Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata, a tree native to China used in traditional Chinese medicine. It has been used clinically more recently in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT analogues have been approved and are used in cancer chemotherapy today, topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin has also been found in other plants including Chonemorpha fragrans.

Tetrahydroisoquinoline (TIQ or THIQ) is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic solvents. The tetrahydroisoquinoline skeleton is encountered in a number of bioactive compounds and drugs.

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.
Many major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate catalytic activities has an enormous therapeutic effect. Hence, inhibition of the HIV protease is one of the most important approaches for the therapeutic intervention in HIV infection and their development is regarded as major success of structure-based drug design. They are highly effective against HIV and have, since the 1990s, been a key component of anti-retroviral therapies for HIV/AIDS.
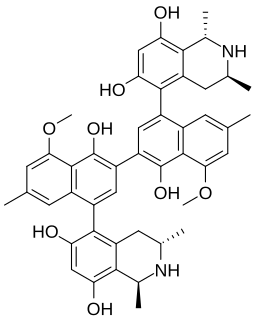
Michellamines are a group of atropisomeric alkaloid which have been found to be HIV viral replication inhibitors in vitro. It was discovered in the leaves of Ancistrocladus korupensis. There are three michellamines represented as A, B, and C; however, michellamine B is the most active against the NID-DZ strain of HIV-2.
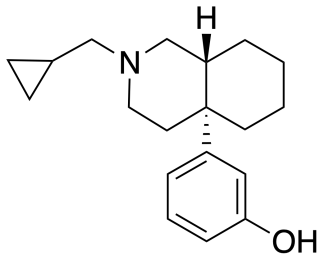
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol, with a number of other related compounds known. Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.

Erysodienone is a key precursor in the biosynthesis of many Erythrina-produced alkaloids. Early work was done by Derek Barton and co-workers to illustrate the biosynthetic pathways towards erythrina alkaloids. It was demonstrated that erysodienone could be synthesized from simple starting materials by a similar approach as its biosynthetic pathway, which led to the development of the biomimetic synthesis of erysodienone.
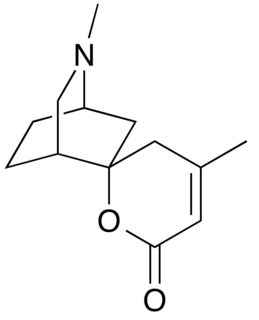
Dioscorine is an alkaloid toxin isolated from the tubers of tropical yam on several continents. It has been used as a monkey poison in some African countries, and as an arrow poison to aid in hunting in several parts of Asia. It was first isolated from Dioscorea hirsute by Boorsma in 1894 and obtained in a crystalline form by Schutte in 1897, and has since been found in other Dioscorea species. Dioscorine is a neurotoxin that acts by blocking the nicotinic acetylcholine receptor. Dioscorine is generally isolated in tandem with other alkaloids such as dioscin but is usually the most potent toxin in the mixture. It is a convulsant, producing symptoms similar to picrotoxin, with which it shares a similar mechanism of action.
Tuticorin Raghavachari Govindachari FNA, FASc (1915–2001), popularly known as TRG, was an Indian natural product chemist, academic, institution builder and the principal of Presidency College, Chennai. He was known for his studies on the synthesis of isoquinolines and phenanthridines and his contributions in elucidating the structure of several plant constituents. He was an elected fellow of the Indian Academy of Sciences and the Indian National Science Academy and was the nominator of Robert Burns Woodward who won the 1965 Nobel Prize in Chemistry. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1960, for his contributions to chemical sciences, making him the first recipient of the award in the chemical sciences category.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Isoquinoline alkaloids are natural products of the group of alkaloids, which are chemically derived from isoquinoline. They form the largest group among the alkaloids.




















