The Actinomycetales is an order of Actinomycetota. A member of the order is often called an actinomycete. Actinomycetales are generally gram-positive and anaerobic and have mycelia in a filamentous and branching growth pattern. Some actinomycetes can form rod- or coccoid-shaped forms, while others can form spores on aerial hyphae. Actinomycetales bacteria can be infected by bacteriophages, which are called actinophages. Actinomycetales can range from harmless bacteria to pathogens with resistance to antibiotics.
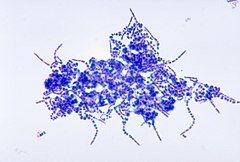
Leuconostoc mesenteroides is a species of lactic acid bacteria associated with fermentation, under conditions of salinity and low temperatures. In some cases of vegetable and food storage, it was associated with pathogenicity. L. mesenteroides is approximately 0.5-0.7 μm in diameter and has a length of 0.7-1.2 μm, producing small grayish colonies that are typically less than 1.0 mm in diameter. It is facultatively anaerobic, Gram-positive, non-motile, non-sporogenous, and spherical. It often forms lenticular coccoid cells in pairs and chains, however, it can occasionally form short rods with rounded ends in long chains, as its shape can differ depending on what media the species is grown on. L. mesenteroides grows best at 30 °C, but can survive in temperatures ranging from 10 °C to 30 °C. Its optimum pH is 5.5, but can still show growth in pH of 4.5-7.0.
Elaeophora schneideri is a nematode which infests several mammalian hosts in North America. It is transmitted by horse-flies. Infection in the normal definitive hosts, mule deer or black-tailed deer, seldom produces clinical symptoms. In other hosts, such as sheep, elk, moose, and goats, infection with E. schneideri leads to elaeophorosis. Symptoms of elaeophorosis include necrosis of the muzzle, ears, and optic nerves; lack of coordination (ataxia); facial or lower limb dermatitis; horn deformities; blindness; and death.
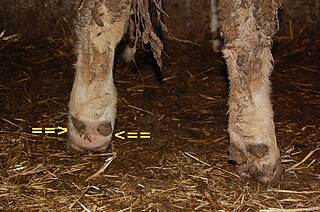
Foot rot, also known as foul-in-the-foot, interdigital necrobacillosis or infectious pododermatitis, is a hoof infection commonly found in sheep, goats, and cattle. As the name suggests, it rots away the foot of the animal, more specifically the area between the two toes of the affected animal. It is extremely painful and contagious. It can be treated with a series of medications, but if not treated, the whole herd can become infected. The cause of the infection in cattle is two species of anaerobic bacteria, Fusobacterium necrophorum and Bacteroides melaninogenicus. Both bacteria are common to the environment in which cattle live, and Fusobacterium is present in the rumen and fecal matter of the cattle. In sheep, F. necrophorum first invades the interdigital skin following damage to the skin, and causes interdigital lesions and slight inflammation. The second stage of the disease is marked by the invasion of the foot by the foot rot bacterium Dichelobacter nodosus, a Gram-negative anaerobe. Usually, an injury to the skin between the hooves allows the bacteria to infect the animal. Another cause of foot rot may be high temperatures or humidity, causing the skin between the hooves to crack and let the bacteria infect the foot. This is one of the reasons foot rot is such a major problem in the summer. Foot rot is easily identifiable by its appearance and foul odor. Treatment is usually with an antibiotic medication, and preventing injury to the feet is the best way to prevent foot rot.
This is a glossary of some of the terms used in phytopathology.
Dichelobacter nodosus, formerly Bacteroides nodosus, is a Gram-negative, obligate anaerobe of the family Cardiobacteriaceae. It has polar fimbriae and is the causative agent of ovine foot rot as well as interdigital dermatitis. It is the lone species in the genus Dichelobacter.
Rain scald is a dermatological disease affecting cattle and horses. Once in the skin, the bacterium Dermatophilus congolensis causes inflammation of the skin as well as the appearance of scabs and lesions.
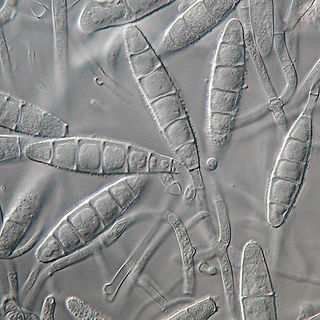
Microsporum gypseum is a soil-associated dermatophyte that occasionally is known to colonise and infect the upper dead layers of the skin of mammals. The name refers to an asexual "form-taxon" that has been associated with four related biological species of fungi: the pathogenic taxa Arthroderma incurvatum, A. gypsea, A. fulva and the non-pathogenic saprotroph A. corniculata. More recent studies have restricted M. gypseum to two teleomorphic species A. gypseum and A. incurvatum. The conidial states of A. fulva and A. corniculata have been assigned to M. fulvum and M. boullardii. Because the anamorphic states of these fungi are so similar, they can be identified reliably only by mating. Two mating strains have been discovered, "+" and "–". The classification of this species has been based on the characteristically rough-walled, blunt, club-shaped, multicelled macroconidia. Synonyms include Achorion gypseum, Microsporum flavescens, M. scorteum, and M. xanthodes. There has been past nomenclatural confusion in the usage of the generic names Microsporum and Microsporon.
Trichophyton concentricum is an anthropophilic dermatophyte believed to be an etiological agent of a type of skin mycosis in humans, evidenced by scaly cutaneous patches on the body known as tinea imbricata. This fungus has been found mainly in the Pacific Islands and South America.

Staphylococcus hyicus is a Gram-positive, facultatively anaerobic bacterium in the genus Staphylococcus. It consists of clustered cocci and forms white circular colonies when grown on blood agar. S. hyicus is a known animal pathogen. It causes disease in poultry, cattle, horses, and pigs. Most notably, it is the agent that causes porcine exudative epidermitis, also known as greasy pig disease, in piglets. S. hyicus is generally considered to not be zoonotic, however it has been shown to be able to cause bacteremia and sepsis in humans.

Mites that infest and parasitize domestic animals cause disease and loss of production. Mites are small invertebrates, most of which are free living but some are parasitic. Mites are similar to ticks and both comprise the order Acari in the phylum Arthropoda. Mites are highly varied and their classification is complex; a simple grouping is used in this introductory article. Vernacular terms to describe diseases caused by mites include scab, mange, and scabies. Mites and ticks have substantially different biology from, and are classed separately from, insects. Mites of domestic animals cause important types of skin disease, and some mites infest other organs. Diagnosis of mite infestations can be difficult because of the small size of most mites, but understanding how mites are adapted to feed within the structure of the skin is useful.
Nannizziopsis vreisii is a keratinophilic microfungus in the Family Onygenaceae of the order Onygenales. Also included in this family are dematophytes and saprophytic species. While the ecology of N. vriessi is not well known, there has been several studies which identifies the Chrysosporium anamorph of N. vriesii as a causal agent of skin lesions in reptiles across several regions. This species is usually identified under a microscope by its white ascomata, and hyaline and globose ascospores. Like many other fungi, N. vreisii has a sexual and asexual state, the asexual states are classified as the genus Chryososporium, Malbranchea or Sporendonema.
Digital dermatitis is a disease that causes lameness in cattle. It was first discovered in Italy in 1974 by Cheli and Mortellaro. This disease is caused by a mixture of different bacteria. Anaerobic bacteria, including spirochetes of the genus Treponema, are found in the lesions associated with the infection. Digital dermatitis is different from foot rot in cattle and both conditions may occur concurrently.
Trueperella pyogenes is a species of nonmotile, facultatively anaerobic, Gram-positive bacteria. The cells typically measure 0.5 by 2.0 μm. They appear as pleomorphic or coccoid rods. They tend to be grouped singly or in short chains but are sometimes grouped into V-shaped pairs.
Malassezia sympodialis is a species in the genus Malassezia. It is characterized by a pronounced lipophily, unilateral, percurrent or sympodial budding and an irregular, corrugated cell wall ultrastructure. It is one of the most common species found on the skin of healthy and diseased individuals. It is considered to be part of the skin's normal human microbiota and begins to colonize the skin of humans shortly after birth. Malassezia sympodialis, often has a symbiotic or commensal relationship with its host, but it can act as a pathogen causing a number of different skin diseases, such as atopic dermatitis.
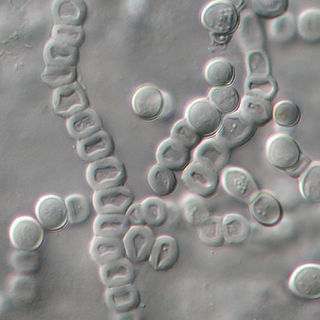
Trichophyton verrucosum, commonly known as the cattle ringworm fungus, is a dermatophyte largely responsible for fungal skin disease in cattle, but is also a common cause of ringworm in donkeys, dogs, goat, sheep, and horses. It has a worldwide distribution, however human infection is more common in rural areas where contact with animals is more frequent, and can cause severe inflammation of the afflicted region. Trichophyton verrucosum was first described by Emile Bodin in 1902.
Chaetomium atrobrunneum is a darkly pigmented mould affiliated with the fungal division, Ascomycota. This species is predominantly saprotrophic, although it has been known to infect animals including humans, showing a proclivity for the tissues of the central nervous system. Chaetomium atrobrunneum was described in 1949 from a mouldy military mattress cover obtained from the island of Guadalcanal.

Actinomyces bovis is a branching, Gram-positive, rod-shaped bacterium of the genus Actinomyces. It is the causative agent of lumpy jaw in cattle, and occasionally causes actinomycosis infections in humans. A. bovis normally populates the gastrointestinal tract of healthy ruminants, but is opportunistic in nature and will move into tissues through ulcerations or abrasions of the mucosa to cause infection. The disease occurs when there is physical damage to the tissue of the mouth, allowing the bacteria to colonize the deep tissue and bone, typically affecting the mandible and maxilla. Actinomycosis is pathognomonic for abscesses containing "sulfur" granules, and its colonies appear basophilic with club-shaped reaction products on a histological preparation. Lumpy jaw is commonly treated with broad-spectrum antibiotics with varying success, and can be a major economic loss for producers in countries where it is endemic. Because this organism is zoonotic, it is a human health concern and can cause granulomas, abscesses, skin lesions, and bronchopneumonia.
Corynebacterium pseudotuberculosis is a Gram-positive bacterium known to infect ruminants, horses, and rarely people. It is a facultative anaerobic organism that is catalase-positive and capable of beta-hemolysis. In small ruminants, C. pseudotuberculosis causes a disease called caseous lymphadenitis, which is characterized by pyogranulomatous abscess formation. In general, the bacterium causes lesions of the skin, lymph nodes, and internal organs. A disease known as ulcerative lymphagenitis can also result from infection with C. pseudotuberculosis in the distal limbs of horses. This bacterium uses the virulence factors phospholipase D and mycolic acid to damage eukaryotic cell walls and resist phagocytic lysosomal degradation, respectively. Infection with this bacterium is often confirmed by bacterial culture of the purulent exudate. Once the diagnosis has been made, treatment of the infection can begin, but this is difficult due to the nature of the organism and the lesions it forms. Specifically, C. pseudotuberculosis is intrinsically resistant to streptomycin, with varying resistance to penicillin and neomycin depending on the strain. It has been shown to be susceptible to ampicillin, gentamicin, tetracycline, lincomycin, and chloramphenicol. Vaccines have also been produced to develop acquired immunity to this infection.
Staphylococcus borealis is a bacterial species, member of the genus Staphylococcus, closely related to Staphylococcus haemolyticus and described in 2020. Its cells are Gram positive, coccoid in shape, with a diameter of 0.65 to 1.23 μm and form clusters. Additionally, they are facultative anaerobic, coagulase negative and catalase positive. The type strain was isolated from human blood culture at the University Hospital of North Norway, in 1997. Four additional strains included in the description were isolated from skin swabs, from healthy volunteers. The genome sequence of the type strain is deposited in DNA Data Bank of Japan, European Nucleotide Archive and GenBank under the accession number JABVEJ000000000.