In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species replaces a functional group within another electron-deficient molecule. The molecule that contains the electrophile and the leaving functional group is called the substrate.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.
Dimethylformamide is an organic compound with the formula (CH3)2N−C(=O)H. Commonly abbreviated as DMF, this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".

The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens (1900–2000). The usage of aprotic solvents gives predominantly Z-alkenes, while protic solvent gives a mixture of E- and Z-alkenes. As an alkene-generating transformation, the Bamford–Stevens reaction has broad utility in synthetic methodology and complex molecule synthesis.
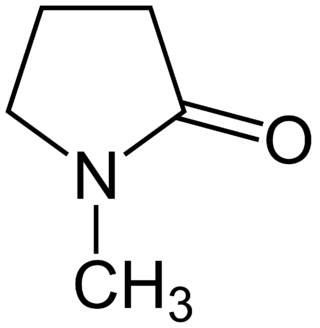
N-Methyl-2-pyrrolidone (NMP) is an organic compound consisting of a 5-membered lactam. It is a colorless liquid, although impure samples can appear yellow. It is miscible with water and with most common organic solvents. It also belongs to the class of dipolar aprotic solvents such as dimethylformamide and dimethyl sulfoxide. It is used in the petrochemical, polymer and battery industries as a solvent, exploiting its nonvolatility and ability to dissolve diverse materials.
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972.

Solvothermal synthesis is a method of producing chemical compounds, in which a solvent containing reagents is put under high pressure and temperature in an autoclave. Many substances dissolve better in the same solvent in such conditions than at standard conditions, enabling reactions that would not otherwise occur and leading to new compounds or polymorphs. Solvothermal synthesis is very similar to the hydrothermal route; both are typically conducted in a stainless steel autoclave. The only difference being that the precursor solution is usually non-aqueous.
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha. It has an ethereous odor. Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.
In chemistry, solvent effects are the influence of a solvent on chemical reactivity or molecular associations. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.

Hydrogen auto-transfer, also known as borrowing hydrogen, is the activation of a chemical reaction by temporary transfer of two hydrogen atoms from the reactant to a catalyst and return of those hydrogen atoms back to a reaction intermediate to form the final product. Two major classes of borrowing hydrogen reactions exist: (a) those that result in hydroxyl substitution, and (b) those that result in carbonyl addition. In the former case, alcohol dehydrogenation generates a transient carbonyl compound that is subject to condensation followed by the return of hydrogen. In the latter case, alcohol dehydrogenation is followed by reductive generation of a nucleophile, which triggers carbonyl addition. As borrowing hydrogen processes avoid manipulations otherwise required for discrete alcohol oxidation and the use of stoichiometric organometallic reagents, they typically display high levels of atom-economy and, hence, are viewed as examples of Green chemistry.
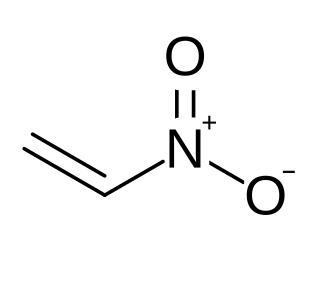
Nitroethylene (also known as nitroethene) is a liquid organic compound with the formula C2H3NO2. It is the simplest nitroalkene, which are unsaturated carbon chains with at least one double bond and a NO2 functional group. Nitroethylene serves as a useful intermediate in the production of various other chemicals.

Cyclopentyl methyl ether (CPME), also known as methoxycyclopentane, is hydrophobic ether solvent. A high boiling point of 106 °C (223 °F) and preferable characteristics such as low formation of peroxides, relative stability under acidic and basic conditions, formation of azeotropes with water coupled with a narrow explosion range render CPME an attractive alternative to other ethereal solvents such as tetrahydrofuran (THF), 2-methyltetrahydrofuran (2-MeTHF), dioxane, and 1,2-dimethoxyethane (DME).

Bis(trimethylsilyl)peroxide (sometimes abbreviated as BTSP) is an organosilicon compound with the formula ((CH3)3SiO)2. It is a colorless liquid that is soluble in organic solvents so long as they lack acidic groups. The compound represents an aprotic analogue of hydrogen peroxide and as such it is used for certain sensitive organic oxidations. Upon treatment with organolithium compounds, it affords the silyl ether.

Levoglucosenone is an organic compound with the formula [OCH2(CH)4CO2]. A pale yellow liquid, it is an unsaturated bicyclic ketone-diether formed from levoglucosan by loss of two molecules of water. As a product of the acid-catalysed pyrolysis of cellulose, D-glucose, and levoglucosan, this liquid hydrocarbon is of interest as a biofuel and biofeedstock.
Chao-Jun "C.-J." Li, a Canadian chemist, is E. B. Eddy Professor of Chemistry and Canada Research Chair in Green Chemistry at McGill University, Montréal. He is known for his pioneering works in Green Solvent and Green Syntheses.

Selin Kara is a Turkish-born chemist and biotechnologist. She is currently a full professor and head of Industrial Biotechnology section at Aarhus University. She studies biocatalysis and has been recognized for her work about deep eutectic solvents and her research regarding cofactor regeneration in biotransformations.
1,6-Hexanediol diglycidyl ether is an organic chemical in the glycidyl ether family. It is an aliphatic compound that is a colorless liquid. It has two epoxide (oxirane) groups per molecule. Its main use is in modifying epoxy resins especially viscosity reduction whilst flexibilizing. It is REACH registered.















