The aldol reaction is a reaction in organic chemistry that combines two carbonyl compounds to form a new β-hydroxy carbonyl compound. Its simplest form might involve the nucleophilic addition of an enolized ketone to another:

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

In organic chemistry, the ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond, in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.
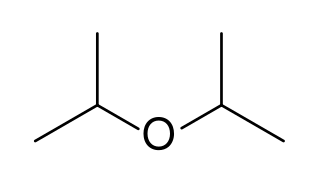
Diisopropyl ether is a secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with organic solvents. It is used as an extractant and an oxygenate gasoline additive. It is obtained industrially as a byproduct in the production of isopropanol by hydration of propylene. Diisopropyl ether is sometimes represented by the abbreviation DIPE.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
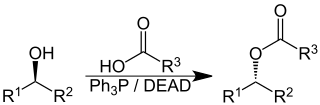
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). In a typical protocol, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine in tetrahydrofuran or other suitable solvent, cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A common side-product is produced when the azodicarboxylate displaces the leaving group instead of the desired nucleophile. This happens if the nucleophile is not acidic enough or is not nucleophilic enough due to steric or electronic constraints. A variation of this reaction utilizing a nitrogen nucleophile is known as a Fukuyama–Mitsunobu.

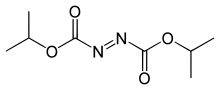
Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2−O−C(=O)−N=N−C(=O)−O−CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles.

Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.
Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by adding four hydrogens. In 2013, its preferred IUPAC name was established as oxane. The compound is a colourless volatile liquid. Derivatives of tetrahydropyran are, however, more common. 2-Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and 3,4-dihydropyran are commonly used as protecting groups in organic synthesis. Furthermore, a tetrahydropyran ring system, i.e., five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.
The aza-Baylis–Hillman reaction or aza-BH reaction in organic chemistry is a variation of the Baylis–Hillman reaction and describes the reaction of an electron deficient alkene, usually an α,β-unsaturated carbonyl compound, with an imine in the presence of a nucleophile. The reaction product is an allylic amine. The reaction can be carried out in enantiomeric excess of up to 90% with the aid of bifunctional chiral BINOL and phosphinyl BINOL compounds, for example in the reaction of n-(4-chloro-benzylidene)-benzenesulfonamide with methyl vinyl ketone (MVK) in cyclopentyl methyl ether and toluene at -15°C.

The Rauhut–Currier reaction, also called the vinylogous Morita–Baylis–Hillman reaction, is an organic reaction describing (in its original scope) the dimerization or isomerization of electron-deficient alkenes such as enones by action of an organophosphine of the type R3P. In a more general description the RC reaction is any coupling of one active alkene / latent enolate to a second Michael acceptor, creating a new C–C bond between the alpha-position of one activated alkene and the beta-position of a second alkene under the influence of a nucleophilic catalyst. The reaction mechanism is essentially that of the related and better known Baylis–Hillman reaction (DABCO not phosphine, carbonyl not enone) but the Rauhut–Currier reaction actually predates it by several years. In comparison to the MBH reaction, the RC reaction lacks substrate reactivity and regioselectivity.

Magnesium iodide is an inorganic compound with the chemical formula MgI2. It forms various hydrates MgI2·xH2O. Magnesium iodide is a salt of magnesium and hydrogen iodide. These salts are typical ionic halides, being highly soluble in water.
Bruce H. Lipshutz is an American chemist. He is a professor at the University of California, Santa Barbara.

Dixanthogen disulfides are a class of organosulfur compounds with the formula (ROC S)2. Usually yellow solids, they are the product of the oxidation of xanthate salts. A common derivative is diethyl dixanthogen disulfide. Diisopropyl dixanthogen disulfide is commercially available. They are structurally related to thiuram disulfides.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective reactions.
In organic chemistry, the Baylis–Hillman, Morita–Baylis–Hillman, or MBH reaction is a carbon-carbon bond-forming reaction between an activated alkene and a carbon electrophile in the presence of a nucleophilic catalyst, such as a tertiary amine or phosphine. The product is densely functionalized, joining the alkene at the α-position to a reduced form of the electrophile.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.

Diethyl oxomalonate is the diethyl ester of mesoxalic acid (ketomalonic acid), the simplest oxodicarboxylic acid and thus the first member (n = 0) of a homologous series HOOC–CO–(CH2)n–COOH with the higher homologues oxalacetic acid (n = 1), α-ketoglutaric acid (n = 2) and α-ketoadipic acid (n = 3) (the latter a metabolite of the amino acid lysine). Diethyl oxomalonate reacts because of its highly polarized keto group as electrophile in addition reactions and is a highly active reactant in pericyclic reactions such as the Diels-Alder reactions, cycloadditions or ene reactions. At humid air, mesoxalic acid diethyl ester reacts with water to give diethyl mesoxalate hydrate and the green-yellow oil are spontaneously converted to white crystals.

Dimethylaziridine carboxylic acid is a naturally occurring non-proteinogenic amino acid and an aziridine. It is found in pleurocybella porrigens and has been responsible for mushroom poisoning in the past.