Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to form an alkene using a titanium chloride compound such as titanium(III) chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the use of a mixture TiCl3 and LiAlH4, which produces the active reagents. Related species have been developed involving the combination of TiCl3 or TiCl4 with various other reducing agents, including potassium, zinc, and magnesium. This reaction is related to the Pinacol coupling reaction which also proceeds by reductive coupling of carbonyl compounds.

Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as a source of CH2 group. Its behavior is akin to the less convenient reagent diazomethane.

The Petasis reagent, named after Nicos A. Petasis, is an organotitanium compound with the formula Cp2Ti(CH3)2. It is an orange-colored solid.

Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.

Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, perfumes and other organic compounds. Allyl bromide is a colorless liquid, although commercial samples appear yellow or brown. It is an irritant and a potentially dangerous alkylating agent. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.
Benzeneselenol, also known as selenophenol, is the organoselenium compound with the chemical formula C6H5SeH, often abbreviated PhSeH. It is the selenium analog of phenol. This colourless, malodorous compound is a reagent in organic synthesis.

Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This yellow-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.
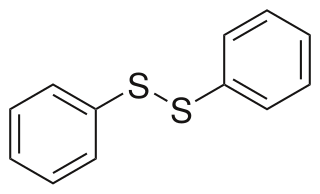
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiophenol is responsible for the disagreeable odour associated with this compound.
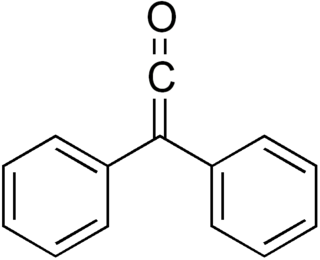
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.
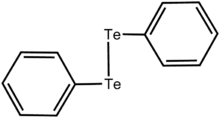
Organotellurium chemistry describes the synthesis and properties of organotellurium compounds, chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of it having few applications.
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6H5SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these similarities. Phenylsilane is soluble in organic solvents.

Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. It has been used as a reducing agent in organic chemistry. It is a rare example of a compound with phosphorus in the +2 oxidation state, and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides.

2,6-Di-tert-butylpyridine is an organic compound with the formula (Me3C)2C5H3N. This colourless, oily liquid is derived from pyridine by replacement of the two H atoms with tert-butyl groups. It is a hindered base. For example, it can be protonated, but it does not form an adduct with boron trifluoride.

Diphenylzinc is an organozinc compound. It is commonly used as the synthetic equivalent of a Ph− synthon. Solvent-free diphenylzinc exists as dimeric PhZn(μ-Ph)2ZnPh molecules in the solid state.
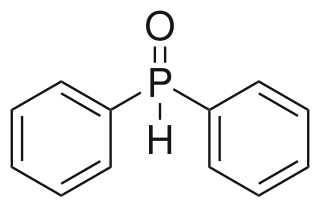
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.

PhSeZn-halides is a class of chemical compounds with general formula PhSeZnX. This is the first example of bench stable zinc selenolates with a remarkable nucleophilic reactivity under on-water conditions. The synthesis and application of these reagents were first reported by Dr. Claudio Santi of the University of Perugia in 2008. In 2016, the lead compound (PhSeZnCl) becomes commercially available.

Bromopentafluorobenzene is an organofluorine compound with the formula C6F5Br. It is a colorless liquid that is used to prepare pentafluophenyl compounds. These syntheses typically proceed via the intermediacy of C6F5Li or the Grignard reagent. Illustrative is preparation of tris(pentafluorophenyl)borane:

Isopropylmagnesium chloride is an organometallic compound with the general formula (CH3)2HCMgCl. This highly flammable, colorless, and moisture sensitive material is the Grignard reagent derived from isopropyl chloride. It is commercially available, usually as a solution in tetrahydrofuran.