
Hereditary haemochromatosis type 1 is a genetic disorder characterized by excessive intestinal absorption of dietary iron, resulting in a pathological increase in total body iron stores. Humans, like most animals, have no means to excrete excess iron, with the exception of menstruation which, for the average woman, results in a loss of 3.2 mg of iron.

Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key component of the hemoglobin protein, acting as a transport medium for electrons within the cells in the form of cytochromes, and facilitating oxygen enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and death.

Iron overload or haemochromatosis indicates increased total accumulation of iron in the body from any cause and resulting organ damage. The most important causes are hereditary haemochromatosis, a genetic disorder, and transfusional iron overload, which can result from repeated blood transfusions.

Iron-deficiency anemia is anemia caused by a lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as feeling tired, weak, short of breath, or having decreased ability to exercise. Anemia that comes on quickly often has more severe symptoms, including confusion, feeling like one is going to pass out or increased thirst. Anemia is typically significant before a person becomes noticeably pale. Children with iron deficiency anemia may have problems with growth and development. There may be additional symptoms depending on the underlying cause.

Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives rise to biliverdin and iron. The body then traps the released iron and stores it as hemosiderin in tissues. Hemosiderin is also generated from the abnormal metabolic pathway of ferritin.

Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia.
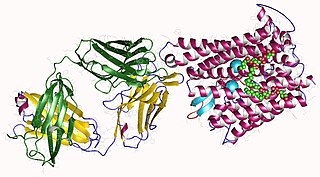
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the SLC40A1 gene, and is part of the Ferroportin (Fpn)Family (TC# 2.A.100). Ferroportin is a transmembrane protein that transports iron from the inside of a cell to the outside of the cell. Ferroportin is the only known iron exporter.
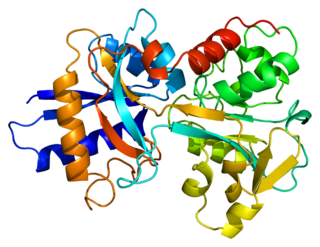
African iron overload, also known as Bantu siderosis or dietary iron overload, is an iron overload disorder first observed among people of African descent in Southern Africa and Central Africa. Dietary iron overload is the consumption of large amount of home-brewed beer with high amount of iron content in it. Preparing beer in iron pots or drums results in high iron content. The iron content in home-brewed beer is around 46–82 mg/L, compared to 0.5 mg/L in commercial beer. Dietary overload was prevalent in both the rural and urban Black African population, with the introduction of commercial beer in urban areas, the condition has decreased. However, the condition is still common in rural areas. Until recently, studies have shown that genetics might play a role in this disorder. Combination of excess iron and functional changes in ferroportin seems to be the probable cause. This disorder can be treated with phlebotomy therapy or iron chelation therapy.

TRPV6 is a membrane calcium (Ca2+) channel protein which is particularly involved in the first step in Ca2+absorption in the intestine.

Hephaestin, also known as HEPH, is a protein which in humans is encoded by the HEPH gene.

Natural resistance-associated macrophage protein 2, also known as divalent metal transporter 1 (DMT1) and divalent cation transporter 1 (DCT1), is a protein that in humans is encoded by the SLC11A2 gene. DMT1 represents a large family of orthologous metal ion transporter proteins that are highly conserved from bacteria to humans.

Iron is an important biological element. It is used in both the ubiquitous Iron-sulfur proteins and in Vertebrates it is used in Hemoglobin which is essential for Blood and oxygen transport.

NADH-cytochrome b5 reductase 3 is an enzyme that in humans is encoded by the CYB5R3 gene.

Cytochrome P450 2S1 is a protein that in humans is encoded by the CYP2S1 gene. The gene is located in chromosome 19q13.2 within a cluster including other CYP2 family members such as CYP2A6, CYP2A13, CYP2B6, and CYP2F1.

Ubiquinol-cytochrome c reductase binding protein, also known as UQCRB, Complex III subunit 7, QP-C, or Ubiquinol-cytochrome c reductase complex 14 kDa protein is a protein which in humans is encoded by the UQCRB gene. This gene encodes a subunit of the ubiquinol-cytochrome c oxidoreductase complex, which consists of one mitochondrial-encoded and 10 nuclear-encoded subunits. Mutations in this gene are associated with mitochondrial complex III deficiency. Alternatively spliced transcript variants have been found for this gene. Related pseudogenes have been identified on chromosomes 1, 5 and X.

Ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1, also known as UQCRFS1, Rieske iron-sulfur (Fe-S) protein, Cytochrome b-c1 complex subunit 5, or Complex III subunit 5 is a protein which in humans is encoded by the UQCRFS1 gene. UQCRFS1 is a subunit of the respiratory chain protein Ubiquinol Cytochrome c Reductase, which consists of the products of one mitochondrially encoded gene, MTCYTB and ten nuclear genes UQCRC1, UQCRC2, Cytochrome C1, UQCRFS1, UQCRB,UQCRQ, UQCRH, UCRC, and UQCR.

Mitochondrial ferritin is a ferroxidase enzyme that in humans is encoded by the FTMT gene.
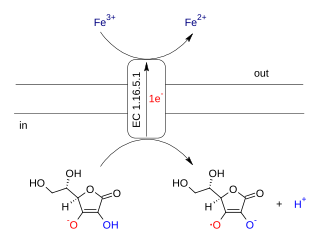
Ascorbate ferrireductase (transmembrane) (EC 1.16.5.1, cytochrome b561) is an enzyme with systematic name Fe(III):ascorbate oxidorectuctase (electron-translocating). This enzyme catalyses the following chemical reaction

Aconitase 1, soluble is a protein that in humans is encoded by the ACO1 gene.
Iron preparation is the formulation for iron supplements indicated in prophylaxis and treatment of iron-deficiency anemia. Examples of iron preparation include ferrous sulfate, ferrous gluconate, and ferrous fumarate. It can be administered orally, and by intravenous injection, or intramuscular injection.






















