In biology, homeostasis is the state of steady internal physical and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and includes many variables, such as body temperature and fluid balance, being kept within certain pre-set limits. Other variables include the pH of extracellular fluid, the concentrations of sodium, potassium, and calcium ions, as well as the blood sugar level, and these need to be regulated despite changes in the environment, diet, or level of activity. Each of these variables is controlled by one or more regulators or homeostatic mechanisms, which together maintain life.

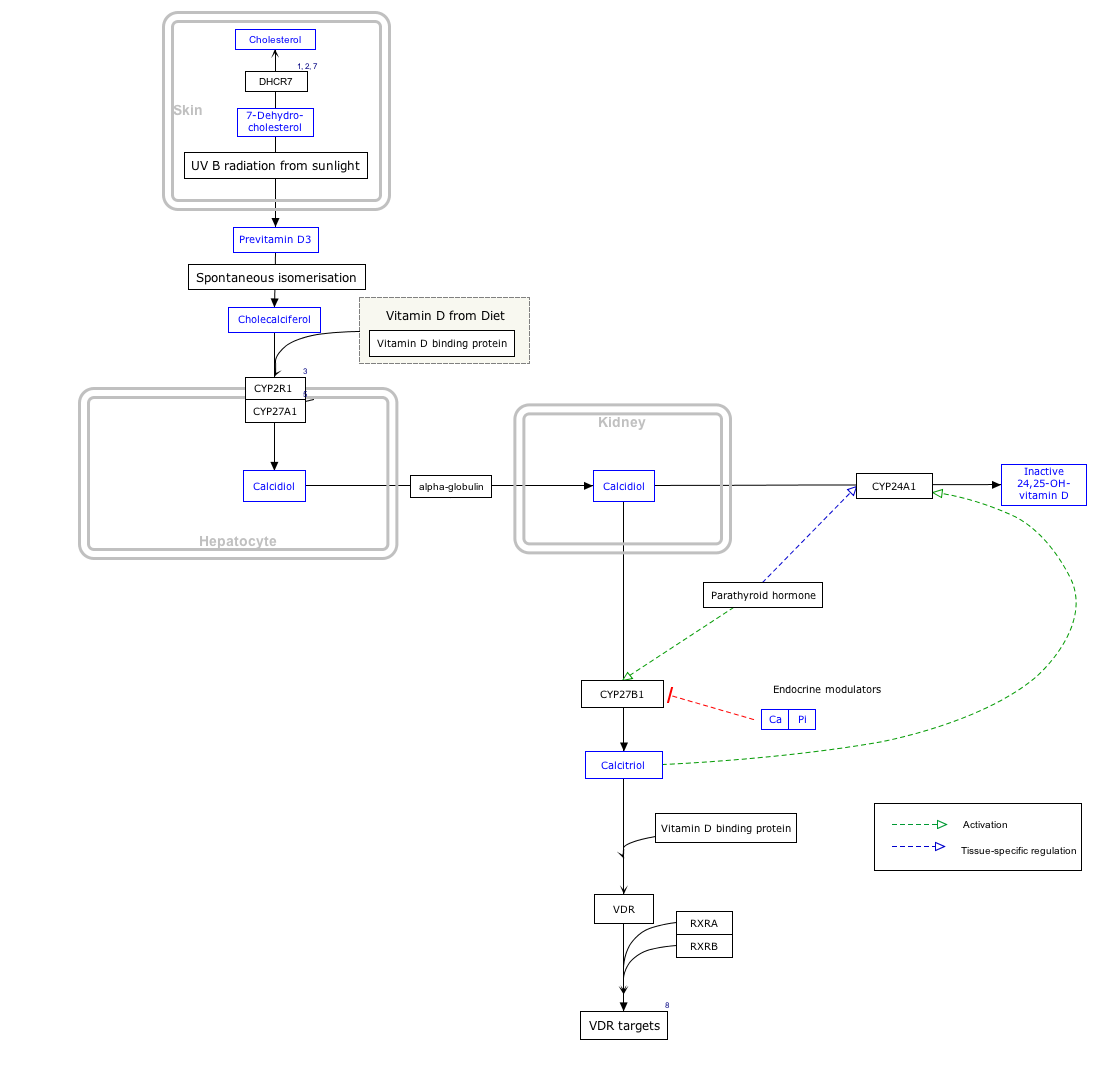
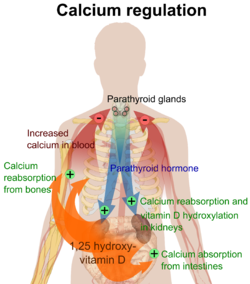
Parathyroid glands are small endocrine glands in the neck of humans and other tetrapods. Humans usually have four parathyroid glands, located on the back of the thyroid gland in variable locations. The parathyroid gland produces and secretes parathyroid hormone in response to low blood calcium, which plays a key role in regulating the amount of calcium in the blood and within the bones.

Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation.

Parathyroid hormone (PTH), also called parathormone or parathyrin, is a peptide hormone secreted by the parathyroid glands that regulates the serum calcium concentration through its effects on bone, kidney, and intestine.

Calcitonin is a 32 amino acid peptide hormone secreted by parafollicular cells (also known as C cells) of the thyroid (or endostyle) in humans and other chordates in the ultimopharyngeal body. It acts to reduce blood calcium (Ca2+), opposing the effects of parathyroid hormone (PTH).

Hypocalcemia is a medical condition characterized by low calcium levels in the blood serum. The normal range of blood calcium is typically between 2.1–2.6 mmol/L, while levels less than 2.1 mmol/L are defined as hypocalcemic. Mildly low levels that develop slowly often have no symptoms. Otherwise symptoms may include numbness, muscle spasms, seizures, confusion, or in extreme cases cardiac arrest.
Hypercalcemia, also spelled hypercalcaemia, is a high calcium (Ca2+) level in the blood serum. The normal range is 2.1–2.6 mmol/L (8.8–10.7 mg/dL, 4.3–5.2 mEq/L), with levels greater than 2.6 mmol/L defined as hypercalcemia. Those with a mild increase that has developed slowly typically have no symptoms. In those with greater levels or rapid onset, symptoms may include abdominal pain, bone pain, confusion, depression, weakness, kidney stones or an abnormal heart rhythm including cardiac arrest.
Disorders of calcium metabolism occur when the body has too little or too much calcium. The serum level of calcium is closely regulated within a fairly limited range in the human body. In a healthy physiology, extracellular calcium levels are maintained within a tight range through the actions of parathyroid hormone, vitamin D and the calcium sensing receptor. Disorders in calcium metabolism can lead to hypocalcemia, decreased plasma levels of calcium or hypercalcemia, elevated plasma calcium levels.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.
Hypoparathyroidism is decreased function of the parathyroid glands with underproduction of parathyroid hormone (PTH). This can lead to low levels of calcium in the blood, often causing cramping and twitching of muscles or tetany, and several other symptoms. It is a very rare disease. The condition can be inherited, but it is also encountered after thyroid or parathyroid gland surgery, and it can be caused by immune system-related damage as well as a number of rarer causes. The diagnosis is made with blood tests, and other investigations such as genetic testing depending on the results. The primary treatment of hypoparathyroidism is calcium and vitamin D supplementation. Calcium replacement or vitamin D can ameliorate the symptoms but can increase the risk of kidney stones and chronic kidney disease. Additionally, medications such as recombinant human parathyroid hormone or teriparatide may be given by injection to replace the missing hormone.

Hyperparathyroidism is an increase in parathyroid hormone (PTH) levels in the blood. This occurs from a disorder either within the parathyroid glands or as response to external stimuli. Symptoms of hyperparathyroidism are caused by inappropriately normal or elevated blood calcium excreted from the bones and flowing into the blood stream in response to increased production of parathyroid hormone. In healthy people, when blood calcium levels are high, parathyroid hormone levels should be low. With long-standing hyperparathyroidism, the most common symptom is kidney stones. Other symptoms may include bone pain, weakness, depression, confusion, and increased urination. Both primary and secondary may result in osteoporosis.
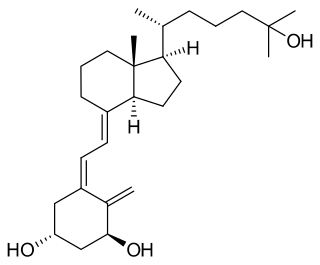
Calcitriol is the active form of vitamin D, normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It is a hormone which binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes. Calcitriol increases blood calcium (Ca2+) mainly by increasing the uptake of calcium from the intestines.

Bone resorption is resorption of bone tissue, that is, the process by which osteoclasts break down the tissue in bones and release the minerals, resulting in a transfer of calcium from bone tissue to the blood.

Secondary hyperparathyroidism is the medical condition of excessive secretion of parathyroid hormone (PTH) by the parathyroid glands in response to hypocalcemia, with resultant hyperplasia of these glands. This disorder is primarily seen in patients with chronic kidney failure. It is sometimes abbreviated "SHPT" in medical literature.
An endocrine bone disease is a bone disease associated with a disorder of the endocrine system. An example is osteitis fibrosa cystica.
Familial hypocalciuric hypercalcemia (FHH) is an inherited condition that can cause hypercalcemia, a serum calcium level typically above 10.2 mg/dL; although uncommon. It is also known as familial benign hypocalciuric hypercalcemia (FBHH) where there is usually a family history of hypercalcemia which is mild, a urine calcium to creatinine ratio <0.01, and urine calcium <200 mg/day.
Recombinant human parathyroid hormone, sold under the brand name Preotact among others, is an artificially manufactured form of the parathyroid hormone used to treat hypoparathyroidism. Recombinant human parathyroid hormone is used in the treatment of osteoporosis in postmenopausal women at high risk of osteoporotic fractures. A significant reduction in the incidence of vertebral fractures has been demonstrated. It is used in combination with calcium and vitamin D supplements.
Chronic kidney disease–mineral and bone disorder (CKD–MBD) is one of the many complications associated with chronic kidney disease. It represents a systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following:

The calcium cycle is a transfer of calcium between dissolved and solid phases. There is a continuous supply of calcium ions into waterways from rocks, organisms, and soils. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate and calcium silicate, which can deposit to form sediments or the exoskeletons of organisms. Calcium ions can also be utilized biologically, as calcium is essential to biological functions such as the production of bones and teeth or cellular function. The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes. Calcium moves through these different media as it cycles throughout the Earth. The marine calcium cycle is affected by changing atmospheric carbon dioxide due to ocean acidification.

Idiopathic hypercalcinuria (IH) is a condition including an excessive urinary calcium level with a normal blood calcium level resulting from no underlying cause. IH has become the most common cause of hypercalciuria and is the most serious metabolic risk factor for developing nephrolithiasis. IH can predispose individuals to osteopenia or osteoporosis, and affects the entire body. IH arises due to faulty calcium homeostasis, a closely monitored process, where slight deviations in calcium transport in the intestines, blood, and bone can lead to excessive calcium excretion, bone mineral density loss, or kidney stone formation. 50%-60% of nephrolithiasis patients suffer from IH and have 5%-15% lower bone density than those who do not.