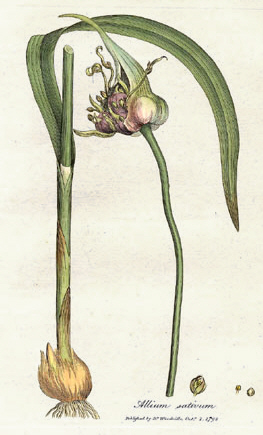
Garlic is a species of bulbous flowering plant in the genus Allium. Its close relatives include the onion, shallot, leek, chive, Welsh onion, and Chinese onion. It is native to South Asia, Central Asia and northeastern Iran and has long been used as a seasoning worldwide, with a history of several thousand years of human consumption and use. It was known to ancient Egyptians and has been used as both a food flavoring and a traditional medicine. China produced 73% of the world's supply of garlic in 2021.

An onion, also known as the bulb onion or common onion, is a vegetable that is the most widely cultivated species of the genus Allium. The shallot is a botanical variety of the onion which was classified as a separate species until 2011. Its close relatives include garlic, scallion, leek, and chive.
Allicin is an organosulfur compound obtained from garlic. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is an antifeedant, i.e. the defense mechanism against attacks by pests on the garlic plant.

Olfactory receptors (ORs), also known as odorant receptors, are chemoreceptors expressed in the cell membranes of olfactory receptor neurons and are responsible for the detection of odorants which give rise to the sense of smell. Activated olfactory receptors trigger nerve impulses which transmit information about odor to the brain. In vertebrates, these receptors are members of the class A rhodopsin-like family of G protein-coupled receptors (GPCRs). The olfactory receptors form a multigene family consisting of around 400 genes in humans and 1400 genes in mice. In insects, olfactory receptors are members of an unrelated group of ligand-gated ion channels.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

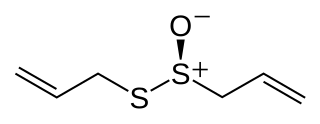
Ajoene is an organosulfur compound found in garlic (Allium sativum) extracts. It is a colorless liquid that contains sulfoxide and disulfide functional groups. The name (and pronunciation) is derived from "ajo", the Spanish word for garlic. It is found as a mixture of up to four stereoisomers, which differ in terms of the stereochemistry of the central alkene (E- vs Z-) and the chirality of the sulfoxide sulfur (R- vs S-).
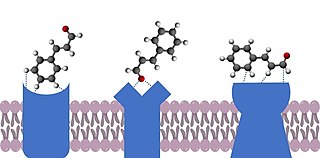
The docking theory of olfaction proposes that the smell of an odorant molecule is due to a range of weak non-covalent interactions between the odorant [a ligand] and one or more G protein-coupled odorant receptors. These include intermolecular forces, such as dipole-dipole and Van der Waals interactions, as well as hydrogen bonding. More specific proposed interactions include metal-ion, ion-ion, cation-pi and pi-stacking. Interactions can be influenced by the hydrophobic effect. Conformational changes can also have a significant impact on interactions with receptors, as ligands have been shown to interact with ligands without being in their conformation of lowest energy.
The vibration theory of smell proposes that a molecule's smell character is due to its vibrational frequency in the infrared range. This controversial theory is an alternative to the more widely accepted docking theory of olfaction, which proposes that a molecule's smell character is due to a range of weak non-covalent interactions between its protein odorant receptor, such as electrostatic and Van der Waals interactions as well as H-bonding, dipole attraction, pi-stacking, metal ion, Cation–pi interaction, and hydrophobic effects, in addition to the molecule's conformation.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Diallyl disulfide is an organosulfur compound derived from garlic and a few other genus Allium plants. Along with diallyl trisulfide and diallyl tetrasulfide, it is one of the principal components of the distilled oil of garlic. It is a yellowish liquid which is insoluble in water and has a strong garlic odour. It is produced during the decomposition of allicin, which is released upon crushing garlic and other plants of the family Alliaceae. Diallyl disulfide has many of the health benefits of garlic, but it is also an allergen causing garlic allergy. Highly diluted, it is used as a flavoring in food. It decomposes in the human body into other compounds such as allyl methyl sulfide.

Stainless steel soap is a piece of stainless steel, in the form of a soap bar or other hand-held shape. Its purported purpose is to neutralize or reduce strong odors such as those from handling garlic, onion, durian, guava, salami, or fish.
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene or the sulfur analog of THF. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT.

In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula R−S−OH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

syn-Propanethial S-oxide (or (Z)-propanethial S-oxide), a member of a class of organosulfur compounds known as thiocarbonyl S-oxides (formerly "sulfines"), is a volatile liquid that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes). The chemical is released from onions, Allium cepa, as they are sliced. The release is due to the breaking open of the onion cells and their releasing enzymes called alliinases, which then break down amino acid sulfoxides, generating sulfenic acids. A specific sulfenic acid, 1-propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, called the lachrymatory factor synthase or LFS, giving syn-propanethial S-oxide. The gas diffuses through the air and, on contact with the eye, it stimulates sensory neurons creating a stinging, painful sensation. Tears are released from the tear glands to dilute and flush out the irritant. A structurally related lachrymatory compound, syn-butanethial S-oxide, C4H8OS, has been found in another genus Allium plant, Allium siculum.

Olfactory receptor 1A1 is a protein that in humans is encoded by the OR1A1 gene.
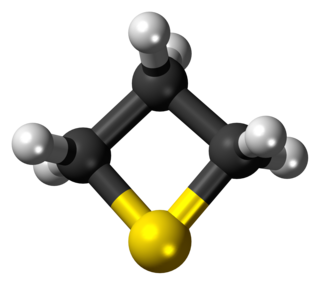
Thietane is a heterocyclic compound containing a saturated four-membered ring with three carbon atoms and one sulfur atom.

Allium stipitatum, Persian shallot, is an Asian species of onion native to central and southwestern Asia.
Vinyldithiins, more precisely named 3-vinyl-4H-1,2-dithiin and 2-vinyl-4H-1,3-dithiin, are organosulfur phytochemicals formed in the breakdown of allicin from crushed garlic (Allium sativum). Vinyldithiins are Diels-Alder dimers of thioacrolein, H2C=CHCH=S, formed in turn by decomposition of allicin. In garlic supplements, vinyldithiins are only found in garlic oil macerates that are made by incubation of crushed garlic in oil.

Allium is a genus of monocotyledonous flowering plants with hundreds of species, including the cultivated onion, garlic, scallion, shallot, leek, and chives. The generic name Allium is the Latin word for garlic, and the type species for the genus is Allium sativum which means "cultivated garlic".

In chemistry, a selenosulfide refers to distinct classes of inorganic and organic compounds containing sulfur and selenium. The organic derivatives contain Se-S bonds, whereas the inorganic derivatives are more variable.















