
Poly(methyl methacrylate) (PMMA) is a synthetic polymer derived from methyl methacrylate. It is a transparent thermoplastic, used as an engineering plastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Hesalite, Plexiglas, Acrylite, Lucite, and Perspex, among several others. This plastic is often used in sheet form as a lightweight or shatter-resistant alternative to glass. It can also be used as a casting resin, in inks and coatings, and for many other purposes.
Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.

Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).
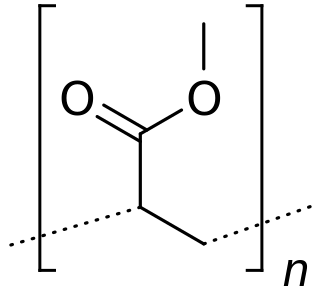
Poly(methyl acrylate) (PMA) is a family of organic polymers with the formula (CH2CHCO2CH3)n. It is a synthetic acrylate polymer derived from methyl acrylate monomer. The polymers are colorless. This homopolymer is far less important than copolymers derived from methyl acrylate and other monomers. PMA is softer than polymethyl methacrylate (PMMA), It is tough, leathery, and flexible.
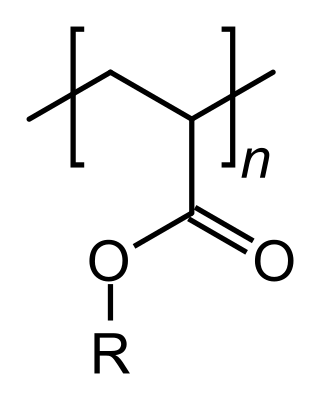
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Methacrylates are derivatives of methacrylic acid. These derivatives are mainly used to make poly(methyl methacrylate) and related polymers.

An acrylic resin is a thermoplastic or thermosetting plastic substance typically derived from acrylic acid, methacrylic acid and acrylate monomers such as butyl acrylate and methacrylate monomers such as methyl methacrylate. Thermoplastic acrylics designate a group of acrylic resins typically containing both a high molecular weight and a high glass transition temperature which exhibit lacquer dry capability. Acrylic resins designed for use in two component systems for crosslinking with isocyanate are referred to as polyols and are made with the monomers previously mentioned as well as hydroxy monomers such as hydroxy ethyl methacrylate. Acrylic resins are produced in different liquid carriers such as a hydrocarbon solvent or water in which case they are referred to as emulsions or dispersions and they are also provided in 100% solids bead form.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.

Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furthermore, in some cases, the comonomers are statistically incorporated within the polymer chain, whereas in other cases, they aggregate. The distribution of comonomers is referred to as the "blockiness" of a copolymer.
Catalytic chain transfer (CCT) is a process that can be incorporated into radical polymerization to obtain greater control over the resulting products.
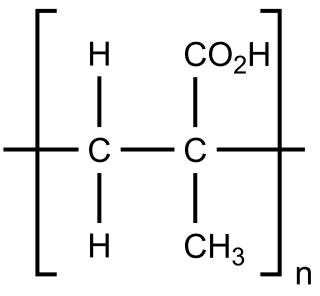
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid (preferred IUPAC name, 2-methylprop-2-enoic acid), which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction (phenolic inhibitors) or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.

Butyl methacrylate is the organic compound with the formula C4H9O2CC(CH3)=CH2. A colorless liquid, it is a common monomer for the preparation of methacrylate polymers. It is typically polymerized under free-radical conditions.

2-Hydroxyisobutyric acid is the organic compound with the formula (CH3)2C(OH)CO2H. A white solid, it is classified as an hydroxycarboxylic acid. It has been considered as a naturally occurring precursor to polyesters. It is closely related to lactic acid.

Poly(ethyl acrylate) (PEA) is a family of organic polymers with the formula (CH2CHCO2CH2CH3)n. It is a synthetic acrylate polymer derived from ethyl acrylate monomer. The polymers are colorless. This homopolymer is far less important than copolymers derived from ethyl acrylate and other monomers. It has a low glass-transition temperature about -8 °C (20 °C).

Poly(butyl acrylate) (PBA) is a family of organic polymers with the formula (CH2CHCO2CH2CH2CH2CH3)n. It is a synthetic acrylate polymer derived from butyl acrylate monomer. The polymers are colorless. This homopolymer is far less important than copolymers derived from methyl acrylate and other monomers. It has a low glass-transition temperature of about -43 °C (20 °C).

Hydroxyethyl acrylate is an organic chemical and an aliphatic compound. It has the formula C5H8O3 and the CAS Registry Number 818–61–1. It is REACH registered with an EU number of 212–454–9. It has dual functionality containing a polymerizable acrylic group and a terminal hydroxy group. It is used to make emulsion polymers along with other monomers and the resultant resins are used in coatings, sealants, adhesives and elastomers and other applications.

tert-Butyl methacrylate is an organic compound with the formula (CH3)3CO2CC(CH3)=CH2. A colorless solid, it is a common monomer for the preparation of methacrylate polymers. It is employed in other kinds of polymerizations.




















