In chemistry, an alcohol, is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugars and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

In organic chemistry, ethers are a class of compounds that contain an ether group—a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group. They have the general formula R−O−R′, where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

Ethanol is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".
Acetaldehyde is an organic chemical compound with the formula CH3CH=O, sometimes abbreviated as MeCH=O. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.

In organic chemistry, an ethyl group is an alkyl substituent with the formula −CH2CH3, derived from ethane. Ethyl is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "eth-" is used to indicate the presence of two carbon atoms in the molecule.

Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.

Ethanethiol, commonly known as ethyl mercaptan, is an organosulfur compound with the formula CH3CH2SH. It is a colorless liquid with a distinct odor. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic in high concentrations. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.
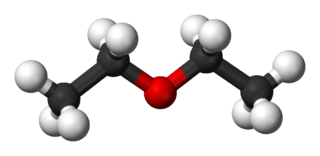
Diethyl ether, or simply ether, is an organic compound with the chemical formula (CH3CH2)2O, sometimes abbreviated as Et2O. It is a colourless, highly volatile, sweet-smelling, extremely flammable liquid. It belongs to the ether class of organic compounds. It is a common solvent. It was formerly used as a general anesthetic.

Charles Adolphe Wurtz was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinions of chemists such as Marcellin Berthelot and Henri Étienne Sainte-Claire Deville. He is well known by organic chemists for the Wurtz reaction, to form carbon-carbon bonds by reacting alkyl halides with sodium, and for his discoveries of ethylamine, ethylene glycol, and the aldol reaction. Wurtz was also an influential writer and educator.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
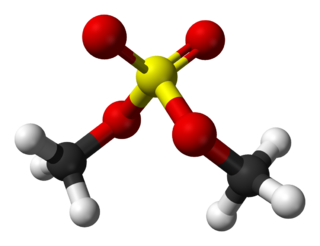
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis. Me2SO4 is a colourless oily liquid with a slight onion-like odour. Like all strong alkylating agents, Me2SO4 is toxic. Its use as a laboratory reagent has been superseded to some extent by methyl triflate, CF3SO3CH3, the methyl ester of trifluoromethanesulfonic acid.

Eilhard Mitscherlich was a German chemist, who is perhaps best remembered today for his discovery of the phenomenon of crystallographic isomorphism in 1819.

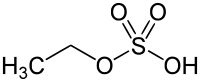
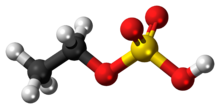
Diethyl sulfate (DES) is an organosulfur compound with the formula (C2H5)2SO4. It occurs as a colorless, oily liquid with a faint peppermint odor. It is toxic, combustible, and likely carcinogenic chemical compound. Diethyl sulfate is used as an ethylating agent.
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
Divinyl ether is the organic compound with the formula O(CH=CH2)2. It is a colorless, volatile liquid that has mainly been of interest as an inhalation anesthetic. It is prepared by treating bis(chloroethyl) ether with base.
Radical theory is an obsolete scientific theory in chemistry describing the structure of organic compounds. The theory was pioneered by Justus von Liebig, Friedrich Wöhler and Auguste Laurent around 1830 and is not related to the modern understanding of free radicals. In this theory, organic compounds were thought to exist as combinations of radicals that could be exchanged in chemical reactions just as chemical elements could be interchanged in inorganic compounds.
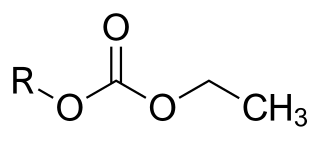
Etabonate or ethyl carbonate is the chemical group with formula –CO
3–C
2H
5, or H
3C–CH
2–O–C(=O)–O–. The names are also used for esters R–OCO
2C
2H
5, for the anion [C
2H
5OCO−
2], and for salts of the latter.