
Carbon is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the few elements known since antiquity.

Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond as a form of carbon is a tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.

A giant planet, sometimes referred to as a jovian planet, is a diverse type of planet much larger than Earth. Giant planets are usually primarily composed of low-boiling point materials (volatiles), rather than rock or other solid matter, but massive solid planets can also exist. There are four such planets in the Solar System: Jupiter, Saturn, Uranus, and Neptune. Many extrasolar giant planets have been identified.

Lonsdaleite, also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found in nature in meteorite debris; when meteors containing graphite strike the Earth, the immense heat and stress of the impact transforms the graphite into diamond, but retains graphite's hexagonal crystal lattice. Lonsdaleite was first identified in 1967 from the Canyon Diablo meteorite, where it occurs as microscopic crystals associated with ordinary diamond.

Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a supercritical phase of matter, which astronomy calls "ice" or volatiles. The planet's atmosphere has a complex layered cloud structure and has the lowest minimum temperature of all the Solar System's planets. It has a marked axial tilt of 82.23° with a retrograde rotation period of 17 hours and 14 minutes. This means that in an 84-Earth-year orbital period around the Sun, its poles get around 42 years of continuous sunlight, followed by 42 years of continuous darkness.
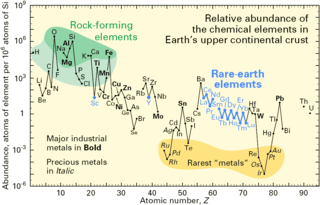
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time.

Presolar grains are interstellar solid matter in the form of tiny solid grains that originated at a time before the Sun was formed. Presolar grains formed within outflowing and cooling gases from earlier presolar stars. The study of presolar grains is typically considered part of the field of cosmochemistry and meteoritics.

Cosmochemistry or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is done primarily through the study of the chemical composition of meteorites and other physical samples. Given that the asteroid parent bodies of meteorites were some of the first solid material to condense from the early solar nebula, cosmochemists are generally, but not exclusively, concerned with the objects contained within the Solar System.

Moissanite is naturally occurring silicon carbide and its various crystalline polymorphs. It has the chemical formula SiC and is a rare mineral, discovered by the French chemist Henri Moissan in 1893. Silicon carbide or moissanite is useful for commercial and industrial applications due to its hardness, optical properties and thermal conductivity.

Carbon is capable of forming many allotropes due to its valency (tetravalent). Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).

A carbon planet is a hypothetical type of planet that contains more carbon than oxygen. Carbon is the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen.

Carbonaceous chondrites or C chondrites are a class of chondritic meteorites comprising at least 8 known groups and many ungrouped meteorites. They include some of the most primitive known meteorites. The C chondrites represent only a small proportion (4.6%) of meteorite falls.

Carbonado, commonly known as black diamond, is one of the toughest forms of natural diamond. It is an impure, high-density, micro-porous form of polycrystalline diamond consisting of diamond, graphite, and amorphous carbon, with minor crystalline precipitates filling pores and occasional reduced metal inclusions. Titanium nitride has been found in carbonado. It is found primarily in alluvial deposits where it is most prominent in mid-elevation equatorial regions such as Central African Republic and in Brazil, where the vast majority of carbonado diamondites have been found. Its natural colour is black or dark grey, and it is more porous than other diamonds.

Cosmic dust – also called extraterrestrial dust, space dust, or star dust – is dust that occurs in outer space or has fallen onto Earth. Most cosmic dust particles measure between a few molecules and 0.1 mm (100 μm), such as micrometeoroids and meteoroids. Cosmic dust can be further distinguished by its astronomical location: intergalactic dust, interstellar dust, interplanetary dust, and circumplanetary dust. There are several methods to obtain space dust measurement.
The study of extraterrestrial atmospheres is an active field of research, both as an aspect of astronomy and to gain insight into Earth's atmosphere. In addition to Earth, many of the other astronomical objects in the Solar System have atmospheres. These include all the giant planets, as well as Mars, Venus and Titan. Several moons and other bodies also have atmospheres, as do comets and the Sun. There is evidence that extrasolar planets can have an atmosphere. Comparisons of these atmospheres to one another and to Earth's atmosphere broaden our basic understanding of atmospheric processes such as the greenhouse effect, aerosol and cloud physics, and atmospheric chemistry and dynamics.

Neptune is the eighth and farthest known planet from the Sun. It is the fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 times the mass of Earth. Compared to its fellow ice giant Uranus, Neptune is slightly more massive, but denser and smaller. Being composed primarily of gases and liquids, it has no well-defined solid surface, and orbits the Sun once every 164.8 years at an orbital distance of 30.1 astronomical units. It is named after the Roman god of the sea and has the astronomical symbol , representing Neptune's trident.

Ureilite is a rare type of stony meteorite that has a unique mineralogical composition very different from that of other stony meteorites. This dark grey or brownish meteorite type is named after the village Novy Urey (Cyrillic: Новый Урей), Mordovia Republic of Russia, where a meteorite of this type fell on 4 September 1886. Notable ureilites are the Novo Urei and the Goalpara, also named for the town in which it landed (Goalpara, Assam India). On 7 October 2008, tiny asteroid 2008 TC3 entered Earth's atmosphere and exploded an estimated 37 kilometres (23 mi) above the Nubian Desert in Sudan. Fragments of this asteroid were recovered the following December and were found to be ureilite. Scientists have discovered amino acids in meteorite 2008 TC3 where none were expected, taking into account high temperatures reached in the explosion of about 1000 °C.
CI chondrites, also called C1 chondrites or Ivuna-type carbonaceous chondrites, are a group of rare carbonaceous chondrite, a type of stony meteorite. They are named after the Ivuna meteorite, the type specimen. CI chondrites have been recovered in France, Canada, India, and Tanzania. Their overall chemical composition closely resembles the elemental composition of the Sun, more so than any other type of meteorite.
The geochemistry of carbon is the study of the transformations involving the element carbon within the systems of the Earth. To a large extent this study is organic geochemistry, but it also includes the very important carbon dioxide. Carbon is transformed by life, and moves between the major phases of the Earth, including the water bodies, atmosphere, and the rocky parts. Carbon is important in the formation of organic mineral deposits, such as coal, petroleum or natural gas. Most carbon is cycled through the atmosphere into living organisms and then respirated back into the atmosphere. However an important part of the carbon cycle involves the trapping of living matter into sediments. The carbon then becomes part of a sedimentary rock when lithification happens. Human technology or natural processes such as weathering, or underground life or water can return the carbon from sedimentary rocks to the atmosphere. From that point it can be transformed in the rock cycle into metamorphic rocks, or melted into igneous rocks. Carbon can return to the surface of the Earth by volcanoes or via uplift in tectonic processes. Carbon is returned to the atmosphere via volcanic gases. Carbon undergoes transformation in the mantle under pressure to diamond and other minerals, and also exists in the Earth's outer core in solution with iron, and may also be present in the inner core.
CM chondrites are a group of chondritic meteorites which resemble their type specimen, the Mighei meteorite. The CM is the most commonly recovered group of the 'carbonaceous chondrite' class of meteorites, though all are rarer in collections than ordinary chondrites.



















