Laudanum is a tincture of opium containing approximately 10% powdered opium by weight. Laudanum is prepared by dissolving extracts from the opium poppy in alcohol (ethanol).

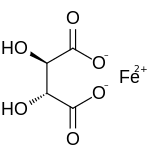
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid.
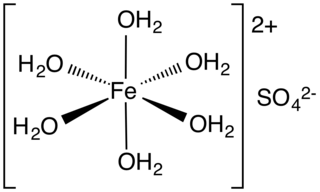
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.
Iron poisoning typically occurs from ingestion of excess iron that results in acute toxicity. Mild symptoms which occur within hours include vomiting, diarrhea, abdominal pain, and drowsiness. In more severe cases, symptoms can include tachypnea, low blood pressure, seizures, or coma. If left untreated, iron poisoning can lead to multi-organ failure resulting in permanent organ damage or death.

A pharmacopoeia, pharmacopeia, or pharmacopoea, in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by the authority of a government or a medical or pharmaceutical society.
ATC code B03Antianemic preparations is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup B03 is part of the anatomical group B Blood and blood forming organs.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Santonin is a drug which was widely used in the past as an anthelminthic. It is an organic compound consisting of colorless flat prisms, turning slightly yellow from the action of light and soluble in alcohol, chloroform and boiling water.
Ethinylestradiol/norethisterone acetate (EE/NETA), or ethinylestradiol/norethindrone acetate, is a combination of ethinylestradiol (EE) and norethisterone acetate (NETA) which is used as birth control and menopausal hormone therapy. EE is an estrogen, while norethisterone acetate (NETA) is a progestin. It is taken by mouth. Some preparations of EE/NETA used in birth control additionally contain an iron supplement in the form of ferrous fumarate.
In metallurgy, non-ferrous metals are metals or alloys that do not contain iron in appreciable amounts.
Iron(II) gluconate, or ferrous gluconate, is a black compound often used as an iron supplement. It is the iron(II) salt of gluconic acid. It is marketed under brand names such as Fergon, Ferralet and Simron.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Iron supplements, also known as iron salts and iron pills, are a number of iron formulations used to treat and prevent iron deficiency including iron deficiency anemia. For prevention they are only recommended in those with poor absorption, heavy menstrual periods, pregnancy, hemodialysis, or a diet low in iron. Prevention may also be used in low birth weight babies. They are taken by mouth, injection into a vein, or injection into a muscle. While benefits may be seen in days, up to two months may be required until iron levels return to normal.

Iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula FeCO
3, that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations Fe2+
and carbonate anions CO2−
3.
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2Fe(SO4)2(H2O)6. Containing two different cations, Fe2+ and NH+4, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Its mineral form is mohrite.
The enzyme D(−)-tartrate dehydratase (EC 4.2.1.81) catalyzes the chemical reaction
Ethinylestradiol/norethisterone (EE/NET), or ethinylestradiol/norethindrone, is a combination birth control pill which contains ethinylestradiol (EE), an estrogen and norethisterone (NET), a progestin. It is used for birth control, symptoms of menstruation, endometriosis, and menopausal symptoms. Other uses include acne. It is taken by mouth. Some preparations of EE/NET additionally contain an iron supplement in the form of ferrous fumarate.

Effervescent or carbon tablets are tablets which are designed to dissolve in water and release carbon dioxide. The carbon dioxide is generated by a reaction of a compound containing bicarbonate such as sodium bicarbonate or magnesium bicarbonate, with an acid such as citric acid or tartaric acid. Both compounds are present in the tablet in powder form and start reacting as soon as they dissolve in water.

Ethinylestradiol/levonorgestrel (EE/LNG) is a combined birth control pill made up of ethinylestradiol, an estrogen and levonorgestrel a progestin. It is used for birth control, symptoms of menstruation, endometriosis, and as emergency contraception. It is taken by mouth. Some preparations of EE/LNG additionally contain an iron supplement in the form of ferrous bisglycinate or ferrous fumarate.
Iron preparation is the formulation for iron supplements indicated in prophylaxis and treatment of iron-deficiency anemia. Examples of iron preparation include ferrous sulfate, ferrous gluconate, and ferrous fumarate. It can be administered orally, and by intravenous injection, or intramuscular injection.