Related Research Articles

Exodeoxyribonuclease V is an enzyme of E. coli that initiates recombinational repair from potentially lethal double strand breaks in DNA which may result from ionizing radiation, replication errors, endonucleases, oxidative damage, and a host of other factors. The RecBCD enzyme is both a helicase that unwinds, or separates the strands of DNA, and a nuclease that makes single-stranded nicks in DNA. It catalyses exonucleolytic cleavage in either 5′- to 3′- or 3′- to 5′-direction to yield 5′-phosphooligonucleotides.

Richard Axel is an American molecular biologist and university professor in the Department of Neuroscience at Columbia University and investigator at the Howard Hughes Medical Institute. His work on the olfactory system won him and Linda Buck, a former postdoctoral research scientist in his group, the Nobel Prize in Physiology or Medicine in 2004.

MyoD, also known as myoblast determination protein 1, is a protein in animals that plays a major role in regulating muscle differentiation. MyoD, which was discovered in the laboratory of Harold M. Weintraub, belongs to a family of proteins known as myogenic regulatory factors (MRFs). These bHLH transcription factors act sequentially in myogenic differentiation. Vertebrate MRF family members include MyoD1, Myf5, myogenin, and MRF4 (Myf6). In non-vertebrate animals, a single MyoD protein is typically found.

Robert G. Roeder is an American biochemist. He is known as a pioneer scientist in eukaryotic transcription. He discovered three distinct nuclear RNA polymerases in 1969 and characterized many proteins involved in the regulation of transcription, including basic transcription factors and the first mammalian gene-specific activator over five decades of research. He is the recipient of the Gairdner Foundation International Award in 2000, the Albert Lasker Award for Basic Medical Research in 2003, and the Kyoto Prize in 2021. He currently serves as Arnold and Mabel Beckman Professor and Head of the Laboratory of Biochemical and Molecular Biology at The Rockefeller University.

Michael Stuart Brown ForMemRS NAS AAA&S APS is an American geneticist and Nobel laureate. He was awarded the Nobel Prize in Physiology or Medicine with Joseph L. Goldstein in 1985 for describing the regulation of cholesterol metabolism.

Myogenin, is a transcriptional activator encoded by the MYOG gene. Myogenin is a muscle-specific basic-helix-loop-helix (bHLH) transcription factor involved in the coordination of skeletal muscle development or myogenesis and repair. Myogenin is a member of the MyoD family of transcription factors, which also includes MyoD, Myf5, and MRF4.
Myogenic regulatory factors (MRF) are basic helix-loop-helix (bHLH) transcription factors that regulate myogenesis: MyoD, Myf5, myogenin, and MRF4.
An E-box is a DNA response element found in some eukaryotes that acts as a protein-binding site and has been found to regulate gene expression in neurons, muscles, and other tissues. Its specific DNA sequence, CANNTG, with a palindromic canonical sequence of CACGTG, is recognized and bound by transcription factors to initiate gene transcription. Once the transcription factors bind to the promoters through the E-box, other enzymes can bind to the promoter and facilitate transcription from DNA to mRNA.

Histone-binding protein RBBP4 is a protein that in humans is encoded by the RBBP4 gene.

Pre-B-cell leukemia transcription factor 1 is a protein that in humans is encoded by the PBX1 gene. The homologous protein in Drosophila is known as extradenticle, and causes changes in embryonic development.

Egl nine homolog 2 is a protein that in humans is encoded by the EGLN2 gene. ELGN2 is an alpha-ketoglutarate-dependent hydroxylase, a superfamily of non-haem iron-containing proteins.

Homeobox protein Hox-B5 is a protein that in humans is encoded by the HOXB5 gene.
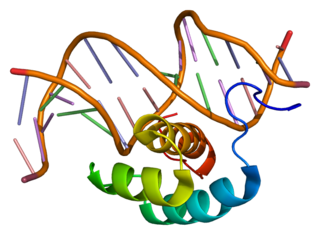
Homeobox protein Hox-A7 is a protein that in humans is encoded by the HOXA7 gene.

MAD protein is a protein that in humans is encoded by the MXD1 gene.
In molecular biology, a displacement loop or D-loop is a DNA structure where the two strands of a double-stranded DNA molecule are separated for a stretch and held apart by a third strand of DNA. An R-loop is similar to a D-loop, but in this case the third strand is RNA rather than DNA. The third strand has a base sequence which is complementary to one of the main strands and pairs with it, thus displacing the other complementary main strand in the region. Within that region the structure is thus a form of triple-stranded DNA. A diagram in the paper introducing the term illustrated the D-loop with a shape resembling a capital "D", where the displaced strand formed the loop of the "D".

MyoD family inhibitor is a protein that in humans is encoded by the MDFI gene.

Notch proteins are a family of type-1 transmembrane proteins that form a core component of the Notch signaling pathway, which is highly conserved in metazoans. The Notch extracellular domain mediates interactions with DSL family ligands, allowing it to participate in juxtacrine signaling. The Notch intracellular domain acts as a transcriptional activator when in complex with CSL family transcription factors. Members of this Type 1 transmembrane protein family share several core structures, including an extracellular domain consisting of multiple epidermal growth factor (EGF)-like repeats and an intracellular domain transcriptional activation domain (TAD). Notch family members operate in a variety of different tissues and play a role in a variety of developmental processes by controlling cell fate decisions. Much of what is known about Notch function comes from studies done in Caenorhabditis elegans (C.elegans) and Drosophila melanogaster. Human homologs have also been identified, but details of Notch function and interactions with its ligands are not well known in this context.

Homeobox protein Hox-A2 is a protein that in humans is encoded by the HOXA2 gene.

Myogenic factor 5 is a protein that in humans is encoded by the MYF5 gene. It is a protein with a key role in regulating muscle differentiation or myogenesis, specifically the development of skeletal muscle. Myf5 belongs to a family of proteins known as myogenic regulatory factors (MRFs). These basic helix loop helix transcription factors act sequentially in myogenic differentiation. MRF family members include Myf5, MyoD (Myf3), myogenin, and MRF4 (Myf6). This transcription factor is the earliest of all MRFs to be expressed in the embryo, where it is only markedly expressed for a few days. It functions during that time to commit myogenic precursor cells to become skeletal muscle. In fact, its expression in proliferating myoblasts has led to its classification as a determination factor. Furthermore, Myf5 is a master regulator of muscle development, possessing the ability to induce a muscle phenotype upon its forced expression in fibroblastic cells.
Margaret Buckingham, is a British developmental biologist working in the fields of myogenesis and cardiogenesis. She is an honorary professor at the Pasteur Institute in Paris and emeritus director in the Centre national de la recherche scientifique (CNRS). She is a member of the European Molecular Biology Organization, the Academia Europaea and the French Academy of Sciences.
References
- 1 2 3 Axel, Richard; Maniatis, Thomas (1995). "Harold Weintraub (1945-1995)". Cell. 81 (3): 317–318. doi: 10.1016/0092-8674(95)90382-8 .
- 1 2 3 Kirschner, Marc (1995). "In memory of Harold Weintraub". Mol Biol Cell. 6 (7): 757–758. doi:10.1091/mbc.6.7.757. PMC 301238 . PMID 16562164.
- 1 2 Alberts, B (1995). "Harold M. Weintraub (1945-1995)". Nature. 375 (6526): 19. doi: 10.1038/375019a0 . PMID 7723834. S2CID 4032295.
- 1 2 3 4 Beers, Carole (April 1, 1995). "Dr. Harold Weintraub loved genes and jeans". The Seattle Times. Retrieved 11 November 2014.
- ↑ "Howard Holtzer, Ph.D. (deceased)". University of Pennsylvania - School of Medicine - Howard Holtzer, Ph.D. University of Pennsylvania. Retrieved 25 November 2014.
- ↑ Weintraub, H; Campbell Gle, M; Holtzer, H (1971). "Primitive erythropoiesis in early chick embryogenesis. I. Cell cycle kinetics and control of cell division". J Cell Biol. 50 (3): 652–668. doi:10.1083/jcb.50.3.652. PMC 2108311 . PMID 5098864.
- ↑ Weintraub, H; Campbell, GL; Holtzer, H (1972). "Identification of a developmental program using bromodeoxyuridine". J Mol Biol. 70 (2): 337–350. doi:10.1016/0022-2836(72)90543-8. PMID 5078575.
- ↑ Silberstein, S. D.; Calhoun, A. H.; Lipton, R. B.; Grosberg, B. M.; Cady, R. K.; Dorlas, S.; Simmons, K. A.; Mullin, C.; Liebler, E. J.; Goadsby, P. J.; Saper, J. R.; EVENT Study Group (2016). "Pub Med Search for Weintraub H*[Author]". Neurology. 87 (5): 529–538. doi:10.1212/WNL.0000000000002918. PMC 4970666 . PMID 27412146.
- ↑ "Harold Weintraub". National Academy of Sciences: Member Directory. Retrieved 22 January 2015.
- 1 2 Saxon, Wolfgang (March 31, 1995). "Harold Weintraub, 49, biologist who studied cell development". New York Times. Retrieved November 11, 2014.
- ↑ Weintraub, H; Groudine, M (1976). "Chromosomal subunits in active genes have an altered conformation". Science. 193 (4256): 848–856. Bibcode:1976Sci...193..848W. doi:10.1126/science.948749. PMID 948749.
- ↑ Groudine, M; Weintraub, H (1975). "Rous sarcoma virus activates embryonic globin genes in chick fibroblasts". Proc Natl Acad Sci USA. 72 (11): 4464–4468. Bibcode:1975PNAS...72.4464G. doi: 10.1073/pnas.72.11.4464 . PMC 388742 . PMID 172910.
- ↑ "Our Scientists". Howard Hughes Medical Institute. Retrieved 18 January 2015.
- ↑ Riley, D; Weintraub, H (1979). "Conservative segregation of parental histones during replication in the presence of cycloheximide". Proc Natl Acad Sci USA. 76 (1): 328–332. Bibcode:1979PNAS...76..328R. doi: 10.1073/pnas.76.1.328 . PMC 382932 . PMID 284348.
- ↑ Weisbrod, S; Weintraub, H (1979). "Isolation of a subclass of nuclear proteins responsible for conferring a DNAse I-sensitive structure on globin chromatin". Proc Natl Acad Sci USA. 76 (2): 630–634. Bibcode:1979PNAS...76..630W. doi: 10.1073/pnas.76.2.630 . PMC 383002 . PMID 284387.
- ↑ Seidman, MM; Levine, AJ; Weintraub, H (1979). "The asymmetric segregation of parental nucleosomes during chromosome replication". Cell. 18 (2): 439–49. doi:10.1016/0092-8674(79)90063-1. PMID 227608. S2CID 12405091.
- ↑ Weisbrod, S; Groudine, M; Weintraub, H (1980). "Interaction of HMG 14 and 17 with actively transcribed genes". Cell. 19 (1): 289–301. doi:10.1016/0092-8674(80)90410-9. PMID 6244103. S2CID 44743817.
- ↑ Harland, R; Weintraub, H (1985). "Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA". J Cell Biol. 101 (3): 1094–1099. doi:10.1083/jcb.101.3.1094. PMC 2113735 . PMID 2411734.
- ↑ Weintraub, HM (1990). "Antisense RNA and DNA". Sci Am. 262 (1): 40–46. Bibcode:1990SciAm.262a..40W. doi:10.1038/scientificamerican0190-40. PMID 1688469.
- ↑ Lassar, AB; Paterson, BM; Weintraub, H (1986). "Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts". Cell. 47 (5): 649–656. doi:10.1016/0092-8674(86)90507-6. PMID 2430720. S2CID 46395399.
- ↑ Tapscott, SJ; Davis, RL; Thayer, MJ; Cheng, PF; Weintraub, H; Lassar, AB (1988). "MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts". Science. 242 (4877): 405–411. Bibcode:1988Sci...242..405T. doi:10.1126/science.3175662. PMID 3175662.
- ↑ Thayer, MJ; Tapscott, SJ; Davis, RL; Wright, WE; Lassar, AB; Weintraub, H (1989). "Positive autoregulation of the myogenic determination gene MyoD1". Cell. 58 (2): 241–248. doi:10.1016/0092-8674(89)90838-6. PMID 2546677. S2CID 30089446.
- ↑ Lassar, AB; Buskin, JN; Lockshon, D; Davis, RL; Apone, S; Hauschka, SD; Weintraub, H (1989). "MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer". Cell. 58 (5): 823–831. doi:10.1016/0092-8674(89)90935-5. PMID 2550138. S2CID 27065049.
- ↑ Krause, M; Fire, A; White-Harrison, S; Weintraub, H; Tapscott, S (1992). "Functional conservation of nematode and vertebrate myogenic regulatory factors". J Cell Sci Suppl. 16: 111–115. doi: 10.1242/jcs.1992.supplement_16.13 . PMID 1338434.
- ↑ Lassar, AB; Thayer, MJ; Overell, RW; Weintraub, H (1989). "Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1". Cell. 58 (4): 659–667. doi:10.1016/0092-8674(89)90101-3. PMID 2548731. S2CID 28627738.
- ↑ Sassoon, D; Lyons, G; Wright, WE; Lin, V; Lassar, A; Weintraub, H; Buckingham, M (1989). "Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis". Nature. 341 (6240): 303–307. Bibcode:1989Natur.341..303S. doi:10.1038/341303a0. PMID 2552320. S2CID 4335995.
- ↑ Benezra, R; Davis, RL; Lockshon, D; Turner, DL; Weintraub, H (1990). "The protein Id: a negative regulator of helix-loop-helix DNA binding proteins". Cell. 61 (1): 49–59. doi:10.1016/0092-8674(90)90214-y. PMID 2156629. S2CID 29514374.
- ↑ Weintraub, H; Hauschka, S; Tapscott, SJ (1991). "The MCK enhancer contains a p53 responsive element". Proc Natl Acad Sci USA. 88 (11): 4570–4571. Bibcode:1991PNAS...88.4570W. doi: 10.1073/pnas.88.11.4570 . PMC 51706 . PMID 1647009.
- ↑ Bengal, E; Ransone, L; Scharfmann, R; Dwarki, VJ; Tapscott, SJ; Weintraub, H; Verma, IM (1992). "Functional antagonism between c-Jun and MyoD proteins: a direct physical association". Cell. 68 (3): 507–519. doi:10.1016/0092-8674(92)90187-h. PMID 1310896. S2CID 44966899.
- ↑ Turner, DL; Weintraub, H (1994). "Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate". Genes Dev. 8 (12): 1434–1447. doi: 10.1101/gad.8.12.1434 . PMID 7926743.
- ↑ Zhuang, Y; Soriano, P; Weintraub, H (1994). "The helix-loop-helix gene E2A is required for B cell formation". Cell. 79 (5): 875–884. doi:10.1016/0092-8674(94)90076-0. PMID 8001124. S2CID 24890636.
- ↑ Blackwell, KT; Weintraub, H (1990). "Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection". Science. 250 (4984): 1104–1110. Bibcode:1990Sci...250.1104B. doi:10.1126/science.2174572. PMID 2174572.
- 1 2 "Weintraub Graduate Student Award". Fred Hutchinson Cancer Research Center: Basic Sciences Division. Retrieved 21 January 2015.
- ↑ "Center hosts 15th Weintraub reunion meeting Nov. 4, 5". Fred Hutchinson Cancer Research Center: Hutch News. 31 October 2011. Retrieved November 28, 2014.