
Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.

5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Hedysarum (sweetvetch) is a genus of the botanical family Fabaceae, consisting of about 200 species of annual or perennial herbs in Asia, Europe, North Africa, and North America.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.
In enzymology, a leucoanthocyanidin reductase (EC 1.17.1.3) (LAR, aka leucocyanidin reductase or LCR) is an enzyme that catalyzes the chemical reaction
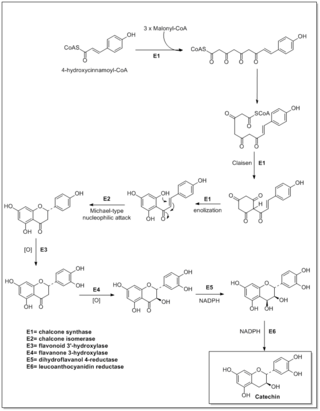
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.

Leucocyanidin is a colorless chemical compound that is a member of the class of natural products known as leucoanthocyanidins.
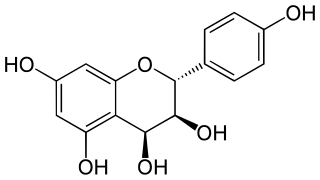
Leucopelargonidin is a colorless chemical compound related to leucoanthocyanins. It can be found in Albizia lebbeck, in the fruit of Anacardium occidentale (Cashew), in the fruit of Areca catechu, in the fruit of Hydnocarpus wightiana, in the rhizome of Rumex hymenosepalus, in Zea mays (Corn) and in Ziziphus jujuba.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.

Procyanidin B2 is a B type proanthocyanidin. Its structure is (−)-Epicatechin-(4β→8)-(−)-epicatechin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.
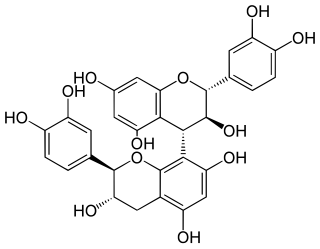
Procyanidin B3 is a B type proanthocyanidin. Procyanidin B3 is a catechin dimer.
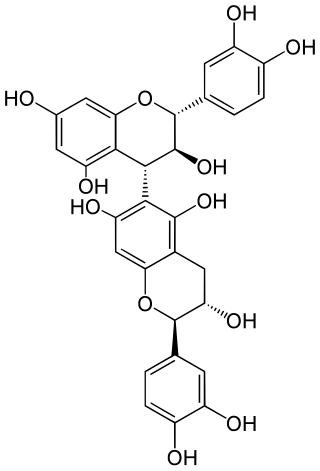
Procyanidin B6 is a B type proanthocyanidin.

Procyanidin C1 (PCC1) is a B type proanthocyanidin. It is an epicatechin trimer found in grape, unripe apples, and cinnamon.

Hedysarum alpinum is a species of flowering plant in the legume family known by the common name alpine sweetvetch. It is called masu in the Iñupiaq language. It has a circumpolar distribution, occurring throughout the northern latitudes of the Northern Hemisphere. In North America it is widespread in Canada and the northernmost United States, including Alaska.
















