
Immunoglobulin A is an antibody that plays a role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined. In absolute terms, between three and five grams are secreted into the intestinal lumen each day. This represents up to 15% of total immunoglobulins produced throughout the body.
Functional gastrointestinal disorders (FGID), also known as disorders of gut–brain interaction, include a number of separate idiopathic disorders which affect different parts of the gastrointestinal tract and involve visceral hypersensitivity and motility disturbances.

Enterochromaffin (EC) cells are a type of enteroendocrine cell, and neuroendocrine cell. They reside alongside the epithelium lining the lumen of the digestive tract and play a crucial role in gastrointestinal regulation, particularly intestinal motility and secretion. They were discovered by Nikolai Kulchitsky.
Gut-associated lymphoid tissue (GALT) is a component of the mucosa-associated lymphoid tissue (MALT) which works in the immune system to protect the body from invasion in the gut.

Paneth cells are cells in the small intestine epithelium, alongside goblet cells, enterocytes, and enteroendocrine cells. Some can also be found in the cecum and appendix. They are located below the intestinal stem cells in the intestinal glands and the large eosinophilic refractile granules that occupy most of their cytoplasm.
Intestinal permeability is a term describing the control of material passing from inside the gastrointestinal tract through the cells lining the gut wall, into the rest of the body. The intestine normally exhibits some permeability, which allows nutrients to pass through the gut, while also maintaining a barrier function to keep potentially harmful substances from leaving the intestine and migrating to the body more widely. In a healthy human intestine, small particles can migrate through tight junction claudin pore pathways, and particles up to 10–15 Å can transit through the paracellular space uptake route. There is some evidence abnormally increased intestinal permeability may play a role in some chronic diseases and inflammatory conditions. The most well understood condition with observed increased intestinal permeability is celiac disease.

In histology, an intestinal gland is a gland found in between villi in the intestinal epithelium lining of the small intestine and large intestine. The glands and intestinal villi are covered by epithelium, which contains multiple types of cells: enterocytes, goblet cells, enteroendocrine cells, cup cells, tuft cells, and at the base of the gland, Paneth cells and stem cells.

Respiratory epithelium, or airway epithelium, is a type of ciliated columnar epithelium found lining most of the respiratory tract as respiratory mucosa, where it serves to moisten and protect the airways. It is not present in the vocal cords of the larynx, or the oropharynx and laryngopharynx, where instead the epithelium is stratified squamous. It also functions as a barrier to potential pathogens and foreign particles, preventing infection and tissue injury by the secretion of mucus and the action of mucociliary clearance.
Microfold cells are found in the gut-associated lymphoid tissue (GALT) of the Peyer's patches in the small intestine, and in the mucosa-associated lymphoid tissue (MALT) of other parts of the gastrointestinal tract. These cells are known to initiate mucosal immunity responses on the apical membrane of the M cells and allow for transport of microbes and particles across the epithelial cell layer from the gut lumen to the lamina propria where interactions with immune cells can take place.
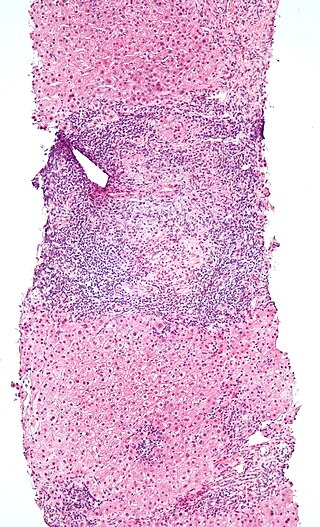
Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract. However, unlike other T cells, IELs do not need priming. Upon encountering antigens, they immediately release cytokines and cause killing of infected target cells. In the GI tract, they are components of gut-associated lymphoid tissue (GALT).
Anti-transglutaminase antibodies (ATA) are autoantibodies against the transglutaminase protein. Antibodies serve an important role in the immune system by detecting cells and substances that the rest of the immune system then eliminates. These cells and substances can be foreign and also can be produced by the body. Antibodies against the body's own products are called autoantibodies. Autoantibodies can sometimes errantly be directed against healthy portions of the organism, causing autoimmune diseases.
Interleukin-22 receptor subunit alpha-2 (IL-22RA2), also known as interleukin-22 binding protein (IL-22BP) is a naturally secreted monomeric protein acting as an interleukin-22 (IL-22) antagonist with inhibitory effects on IL-22 activity in vivo. IL-22BP is in humans encoded by the IL22RA2 gene located on chromosome 6, and in mice is encoded by the il22ra2 gene located on chromosome 10. IL-22BP belongs to the class II cytokine receptor family and it is a soluble receptor homolog of IL-22R.

The intestinal epithelium is the single cell layer that form the luminal surface (lining) of both the small and large intestine (colon) of the gastrointestinal tract. Composed of simple columnar epithelial cells, it serves two main functions: absorbing useful substances into the body and restricting the entry of harmful substances. As part of its protective role, the intestinal epithelium forms an important component of the intestinal mucosal barrier. Certain diseases and conditions are caused by functional defects in the intestinal epithelium. On the other hand, various diseases and conditions can lead to its dysfunction which, in turn, can lead to further complications.

Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract, and the respiratory system. The mucous membranes are in constant contact with microorganisms, food, and inhaled antigens. In healthy states, the mucosal immune system protects the organism against infectious pathogens and maintains a tolerance towards non-harmful commensal microbes and benign environmental substances. Disruption of this balance between tolerance and deprivation of pathogens can lead to pathological conditions such as food allergies, irritable bowel syndrome, susceptibility to infections, and more.
Mucosal-associated invariant T cells make up a subset of T cells in the immune system that display innate, effector-like qualities. In humans, MAIT cells are found in the blood, liver, lungs, and mucosa, defending against microbial activity and infection. The MHC class I-like protein, MR1, is responsible for presenting bacterially-produced vitamin B2 and B9 metabolites to MAIT cells. After the presentation of foreign antigen by MR1, MAIT cells secrete pro-inflammatory cytokines and are capable of lysing bacterially-infected cells. MAIT cells can also be activated through MR1-independent signaling. In addition to possessing innate-like functions, this T cell subset supports the adaptive immune response and has a memory-like phenotype. Furthermore, MAIT cells are thought to play a role in autoimmune diseases, such as multiple sclerosis, arthritis and inflammatory bowel disease, although definitive evidence is yet to be published.
Limosilactobacillus mucosae is a rod shaped species of lactic acid bacteria first isolated from pig intestines. It has mucus-binding activity. The species is an obligate anaerobe, catalase-negative, doesn't form spores and is non-motile. Its type strain is S32T, and has been found to be most closely related to Limosilactobacillus reuteri.
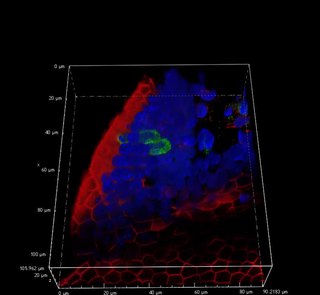
Tuft cells are chemosensory cells in the epithelial lining of the intestines. Similar tufted cells are found in the respiratory epithelium where they are known as brush cells. The name "tuft" refers to the brush-like microvilli projecting from the cells. Ordinarily there are very few tuft cells present but they have been shown to greatly increase at times of a parasitic infection. Several studies have proposed a role for tuft cells in defense against parasitic infection. In the intestine, tuft cells are the sole source of secreted interleukin 25 (IL-25).
Larazotide is a synthetic eight amino acid peptide that functions as a tight junction regulator and reverses leaky junctions to their normally closed state. It is being studied in people with celiac disease.

Type 3 innate lymphoid cells (ILC3) are immune cells from the lymphoid lineage that are part of the innate immune system. These cells participate in innate mechanisms on mucous membranes, contributing to tissue homeostasis, host-commensal mutualism and pathogen clearance. They are part of a heterogeneous group of innate lymphoid cells, which is traditionally divided into three subsets based on their expression of master transcription factors as well as secreted effector cytokines - ILC1, ILC2 and ILC3.











