
Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
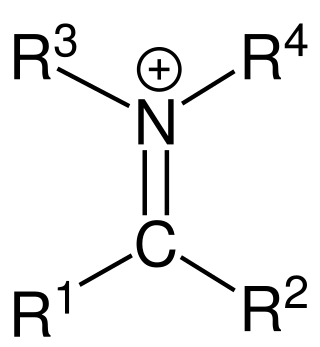
In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.
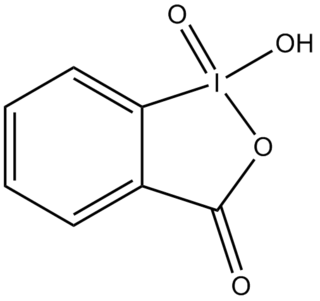
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric acid. Frigerio and co-workers have also demonstrated, in 1999 that potassium bromate may be replaced by commercially available Oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. In the past, it was believed that IBX was shock sensitive, but it was later proposed that samples of IBX were shock sensitive due to the residual potassium bromate left from its preparation. Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
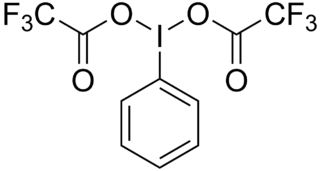
(Bis iodo)benzene, C
6H
5I(OCOCF
3)
2, is a hypervalent iodine compound used as a reagent in organic chemistry. It can be used to carry out the Hofmann rearrangement under acidic conditions.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.
Unlike its lighter congeners, the halogen iodine forms a number of stable organic compounds, in which iodine exhibits higher formal oxidation states than -1 or coordination number exceeding 1. These are the hypervalent organoiodines, often called iodanes after the IUPAC rule used to name them.
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early transition metals such as titanium, niobium, and tungsten. Oxophilicity is often stated to be related to the hardness of the element, within the HSAB theory, but it has been shown that oxophilicity depends more on the electronegativity and effective nuclear charge of the element than on its hardness. This explains why the early transition metals, whose electronegativities and effective nuclear charges are low, are very oxophilic. Many main group compounds are also oxophilic, such as derivatives of aluminium, silicon, and phosphorus(III). The handling of oxophilic compounds often requires air-free techniques.
Iodobenzene dichloride (PhICl2) is a complex of iodobenzene with chlorine. As a reagent for organic chemistry, it is used as an oxidant and chlorinating agent.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.
Carbonyl oxidation with hypervalent iodine reagents involves the functionalization of the α position of carbonyl compounds through the intermediacy of a hypervalent iodine(III) enolate species. This electrophilic intermediate may be attacked by a variety of nucleophiles or undergo rearrangement or elimination.
Phenol oxidation with hypervalent iodine reagents leads to the formation of quinone-type products or iodonium ylides, depending on the structure of the phenol. Trapping of either product is possible with a suitable reagent, and this method is often employed in tandem with a second process.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notable pharmaceutical compounds have a trifluoromethyl group incorporated: fluoxetine, mefloquine, Leflunomide, nulitamide, dutasteride, bicalutamide, aprepitant, celecoxib, fipronil, fluazinam, penthiopyrad, picoxystrobin, fluridone, norflurazon, sorafenib and triflurazin. A relevant agrochemical is trifluralin. The development of synthetic methods for adding trifluoromethyl groups to chemical compounds is actively pursued in academic research.

Trifluoroperacetic acid is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.

(Diacetoxyiodo)benzene, also known as phenyliodine(III) diacetate (PIDA) is a hypervalent iodine chemical with the formula C
6H
5I(OCOCH
3)
2. It is used as an oxidizing agent in organic chemistry.

Togni reagent II is a chemical compound used in organic synthesis for direct electrophilic trifluoromethylation.















