Related Research Articles
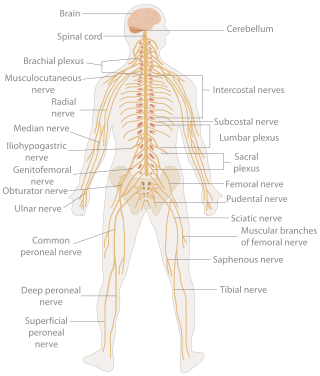
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes that impact the body, then works in tandem with the endocrine system to respond to such events. Nervous tissue first arose in wormlike organisms about 550 to 600 million years ago. In vertebrates it consists of two main parts, the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord. The PNS consists mainly of nerves, which are enclosed bundles of the long fibers or axons, that connect the CNS to every other part of the body. Nerves that transmit signals from the brain are called motor nerves or efferent nerves, while those nerves that transmit information from the body to the CNS are called sensory nerves or afferent. Spinal nerves are mixed nerves that serve both functions. The PNS is divided into three separate subsystems, the somatic, autonomic, and enteric nervous systems. Somatic nerves mediate voluntary movement. The autonomic nervous system is further subdivided into the sympathetic and the parasympathetic nervous systems. The sympathetic nervous system is activated in cases of emergencies to mobilize energy, while the parasympathetic nervous system is activated when organisms are in a relaxed state. The enteric nervous system functions to control the gastrointestinal system. Both autonomic and enteric nervous systems function involuntarily. Nerves that exit from the cranium are called cranial nerves while those exiting from the spinal cord are called spinal nerves.

A circadian rhythm, or circadian cycle, is a natural oscillation that repeats roughly every 24 hours. Circadian rhythms can refer to any process that originates within an organism and responds to the environment. Circadian rhythms are regulated by a circadian clock whose primary function is to rhythmically co-ordinate biological processes so they occur at the correct time to maximise the fitness of an individual. Circadian rhythms have been widely observed in animals, plants, fungi and cyanobacteria and there is evidence that they evolved independently in each of these kingdoms of life.

The suprachiasmatic nucleus or nuclei (SCN) is a small region of the brain in the hypothalamus, situated directly above the optic chiasm. It is the principal circadian pacemaker in mammals and is necessary for generating circadian rhythms. Reception of light inputs from photosensitive retinal ganglion cells allow the SCN to coordinate the subordinate cellular clocks of the body and entrain to the environment. The neuronal and hormonal activities it generates regulate many different body functions in an approximately 24-hour cycle.
In vertebrates, a neuroblast or primitive nerve cell is a postmitotic cell that does not divide further, and which will develop into a neuron after a migration phase. In invertebrates such as Drosophila, neuroblasts are neural progenitor cells which divide asymmetrically to produce a neuroblast, and a daughter cell of varying potency depending on the type of neuroblast. Vertebrate neuroblasts differentiate from radial glial cells and are committed to becoming neurons. Neural stem cells, which only divide symmetrically to produce more neural stem cells, transition gradually into radial glial cells. Radial glial cells, also called radial glial progenitor cells, divide asymmetrically to produce a neuroblast and another radial glial cell that will re-enter the cell cycle.

The ventral nerve cord is a major structure of the invertebrate central nervous system. It is the functional equivalent of the vertebrate spinal cord. The ventral nerve cord coordinates neural signaling from the brain to the body and vice versa, integrating sensory input and locomotor output. Because arthropods have an open circulatory system, decapitated insects can still walk, groom, and mate—illustrating that the circuitry of the ventral nerve cord is sufficient to perform complex motor programs without brain input.
Bursicon is an insect hormone which mediates tanning in the cuticle of adult flies.

Ganglion mother cells (GMCs) are cells involved in neurogenesis, in non-mammals, that divide only once to give rise to two neurons, or one neuron and one glial cell or two glial cells, and are present only in the central nervous system. They are also responsible for transcription factor expression. While each ganglion mother cell necessarily gives rise to two neurons, a neuroblast can asymmetrically divide multiple times. GMCs are the progeny of type I neuroblasts. Neuroblasts asymmetrically divide during embryogenesis to create GMCs. GMCs are only present in certain species and only during the embryonic and larval stages of life. Recent research has shown that there is an intermediate stage between a GMC and two neurons. The GMC forms two ganglion cells which then develop into neurons or glial cells. Embryonic neurogenesis has been extensively studied in Drosophila melanogaster embryos and larvae.
Mosaic analysis with a repressible cell marker, or MARCM, is a genetics technique for creating individually labeled homozygous cells in an otherwise heterozygous Drosophila melanogaster. It has been a crucial tool in studying the development of the Drosophila nervous system. This technique relies on recombination during mitosis mediated by FLP-FRT recombination. As one copy of a gene, provided by the balancer chromosome, is often enough to rescue a mutant phenotype, MARCM clones can be used to study a mutant phenotype in an otherwise wildtype animal.
Steven M. Reppert is an American neuroscientist known for his contributions to the fields of chronobiology and neuroethology. His research has focused primarily on the physiological, cellular, and molecular basis of circadian rhythms in mammals and more recently on the navigational mechanisms of migratory monarch butterflies. He was the Higgins Family Professor of Neuroscience at the University of Massachusetts Medical School from 2001 to 2017, and from 2001 to 2013 was the founding chair of the Department of Neurobiology. Reppert stepped down as chair in 2014. He is currently distinguished professor emeritus of neurobiology.
Ronald J. Konopka (1947-2015) was an American geneticist who studied chronobiology. He made his most notable contribution to the field while working with Drosophila in the lab of Seymour Benzer at the California Institute of Technology. During this work, Konopka discovered the period (per) gene, which controls the period of circadian rhythms.
Pigment dispersing factor (pdf) is a gene that encodes the protein PDF, which is part of a large family of neuropeptides. Its hormonal product, pigment dispersing hormone (PDH), was named for the diurnal pigment movement effect it has in crustacean retinal cells upon its initial discovery in the central nervous system of arthropods. The movement and aggregation of pigments in retina cells and extra-retinal cells is hypothesized to be under a split hormonal control mechanism. One hormonal set is responsible for concentrating chromatophoral pigment by responding to changes in the organism's exposure time to darkness. Another hormonal set is responsible for dispersion and responds to the light cycle. However, insect pdf genes do not function in such pigment migration since they lack the chromatophore.

Cycle (cyc) is a gene in Drosophila melanogaster that encodes the CYCLE protein (CYC). The Cycle gene (cyc) is expressed in a variety of cell types in a circadian manner. It is involved in controlling both the sleep-wake cycle and circadian regulation of gene expression by promoting transcription in a negative feedback mechanism. The cyc gene is located on the left arm of chromosome 3 and codes for a transcription factor containing a basic helix-loop-helix (bHLH) domain and a PAS domain. The 2.17 kb cyc gene is divided into 5 coding exons totaling 1,625 base pairs which code for 413 aminos acid residues. Currently 19 alleles are known for cyc. Orthologs performing the same function in other species include ARNTL and ARNTL2.

Michael Morris Rosbash is an American geneticist and chronobiologist. Rosbash is a professor and researcher at Brandeis University and investigator at the Howard Hughes Medical Institute. Rosbash's research group cloned the Drosophila period gene in 1984 and proposed the Transcription Translation Negative Feedback Loop for circadian clocks in 1990. In 1998, they discovered the cycle gene, clock gene, and cryptochrome photoreceptor in Drosophila through the use of forward genetics, by first identifying the phenotype of a mutant and then determining the genetics behind the mutation. Rosbash was elected to the National Academy of Sciences in 2003. Along with Michael W. Young and Jeffrey C. Hall, he was awarded the 2017 Nobel Prize in Physiology or Medicine "for their discoveries of molecular mechanisms controlling the circadian rhythm".

Jeffrey Connor Hall is an American geneticist and chronobiologist. Hall is Professor Emeritus of Biology at Brandeis University and currently resides in Cambridge, Maine.
Proneural genes encode transcription factors of the basic helix-loop-helix (bHLH) class which are responsible for the development of neuroectodermal progenitor cells. Proneural genes have multiple functions in neural development. They integrate positional information and contribute to the specification of progenitor-cell identity. From the same ectodermal cell types, neural or epidermal cells can develop based on interactions between proneural and neurogenic genes. Neurogenic genes are so called because loss of function mutants show an increase number of developed neural precursors. On the other hand, proneural genes mutants fail to develop neural precursor cells.
Hitoshi Okamura is a Japanese scientist who specializes in chronobiology. He is currently a professor of Systems Biology at Kyoto University Graduate School of Pharmaceutical Sciences and the Research Director of the Japan Science Technology Institute, CREST. Okamura's research group cloned mammalian Period genes, visualized clock oscillation at the single cell level in the central clock of the SCN, and proposed a time-signal neuronal pathway to the adrenal gland. He received a Medal of Honor with Purple Ribbon in 2007 for his research and was awarded Aschoff's Ruler for his work on circadian rhythms in rodents. His lab recently revealed the effects of m6A mRNA methylation on the circadian clock, neuronal communications in jet lag, and the role of dysregulated clocks in salt-induced hypertension.
Paul H. Taghert is an American chronobiologist known for pioneering research on the roles and regulation of neuropeptide signaling in the brain using Drosophila melanogaster as a model. He is a professor of neuroscience in the Department of Neuroscience at Washington University in St. Louis.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.

Drosophila circadian rhythm is a daily 24-hour cycle of rest and activity in the fruit flies of the genus Drosophila. The biological process was discovered and is best understood in the species Drosophila melanogaster. Other than normal sleep-wake activity, D. melanogaster has two unique daily behaviours, namely regular vibration during the process of hatching from the pupa, and during mating. Locomotor activity is maximum at dawn and dusk, while eclosion is at dawn.

Eclosion assays are experimental procedures used to study the process of eclosion in insects, particularly in the model organism drosophila. Eclosion is the process in which an adult insect emerges from its pupal case, or a larval insect hatches from its egg. In holometabolous insects, the circadian clock regulates the timing of adult emergence. The daily rhythm of adult emergence in these insects was among the first circadian rhythms to be investigated. The circadian clock in these insects enforces a daily pattern of emergence by permitting or triggering eclosion during specific time frames and preventing emergence during other periods.
References
- 1 2 3 4 5 6 7 "Jim Truman | Janelia Research Campus". www.janelia.org. Retrieved April 11, 2019.
- ↑ "Researchers at FHL | Friday Harbor Laboratories" . Retrieved April 25, 2019.
- ↑ Žitňan, Dušan & Daubnerová, Ivana. (2016). Chapter 76 "Eclosion Hormone", Editors: Yoshio Takei, Hironori Ando, Kazuyoshi Tsutsui, Handbook of Hormones, Academic Press, Pages 459-e76-2, ISBN 9780128010280. doi : 10.1016/B978-0-12-801028-0.00076-3
- 1 2 3 Saunders, D. S. (2002-10-28). Insect Clocks (3 ed.). Elsevier. ISBN 9780080534718.
- ↑ Tomioka, Kenji; Matsumoto, Akira (December 25, 2009). "A comparative view of insect circadian clock systems". Cellular and Molecular Life Sciences. 67 (9): 1397–1406. doi:10.1007/s00018-009-0232-y. PMID 20035363. S2CID 6547927.
- ↑ Taghert, Paul H.; Nitabach, Michael N. (October 2012). "Peptide Neuromodulation in Invertebrate Model Systems". Neuron. 76 (1): 82–97. doi:10.1016/j.neuron.2012.08.035. PMC 3466441 . PMID 23040808.
- ↑ Venken, Koen J.T.; Simpson, Julie H.; Bellen, Hugo J. (October 2011). "Genetic Manipulation of Genes and Cells in the Nervous System of the Fruit Fly". Neuron. 72 (2): 202–230. doi:10.1016/j.neuron.2011.09.021. PMC 3232021 . PMID 22017985.
- ↑ Weeks, J. C.; Levine, R. B. (1990). "Postembryonic Neuronal Plasticity and its Hormonal Control During Insect Metamorphosis". Annual Review of Neuroscience. 13 (1): 183–194. doi:10.1146/annurev.ne.13.030190.001151. PMID 2183673.
- ↑ Sokol, Nicholas S. (August 2012). "Small temporal RNAs in animal development". Current Opinion in Genetics & Development. 22 (4): 368–373. doi:10.1016/j.gde.2012.04.001. PMC 3419770 . PMID 22578317.
- ↑ Kim, Dongwook W.; Hirth, Frank (October 2009). "Genetic mechanisms regulating stem cell self-renewal and differentiation in the central nervous system of Drosophila". Cell Adhesion & Migration. 3 (4): 402–411. doi:10.4161/cam.3.4.8690. ISSN 1933-6926. PMC 2802756 . PMID 19421003.
- ↑ "Newcomb Cleveland Prize Recipients". American Association for the Advancement of Science. Retrieved 2019-04-25.
- ↑ "John Simon Guggenheim Foundation | All Fellows". Archived from the original on 2019-03-22. Retrieved 2019-04-25.
- ↑ "Winners of the ESA Founders' Memorial Award". Entomological Sociert of America. Retrieved 2019-04-11.
- ↑ "The Wigglesworth Memorial Lecture and Award". Royal Entomological Society. 2017-05-26. Retrieved 2019-04-25.
- ↑ "James William Truman". American Academy of Arts & Sciences. Retrieved 2019-04-25.
- ↑ "2022 NAS Election". www.nasonline.org. Retrieved 2022-05-13.