Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

A mass spectrum is a histogram plot of intensity vs. mass-to-charge ratio (m/z) in a chemical sample, usually acquired using an instrument called a mass spectrometer. Not all mass spectra of a given substance are the same; for example, some mass spectrometers break the analyte molecules into fragments; others observe the intact molecular masses with little fragmentation. A mass spectrum can represent many different types of information based on the type of mass spectrometer and the specific experiment applied. Common fragmentation processes for organic molecules are the McLafferty rearrangement and alpha cleavage. Straight chain alkanes and alkyl groups produce a typical series of peaks: 29 (CH3CH2+), 43 (CH3CH2CH2+), 57 (CH3CH2CH2CH2+), 71 (CH3CH2CH2CH2CH2+) etc.

Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more mass analyzers are coupled together using an additional reaction step to increase their abilities to analyse chemical samples. A common use of tandem MS is the analysis of biomolecules, such as proteins and peptides.

An ion trap is a combination of electric and/or magnetic fields used to capture charged particles — known as ions — often in a system isolated from an external environment. Atomic and molecular ion traps have a number of applications in physics and chemistry such as precision mass spectrometry, improved atomic frequency standards, and quantum computing. In comparison to neutral atom traps, ion traps have deeper trapping potentials that do not depend on the internal electronic structure of a trapped ion. This makes ion traps more suitable for the study of light interactions with single atomic systems. The two most popular types of ion traps are the Penning trap, which forms a potential via a combination of static electric and magnetic fields, and the Paul trap which forms a potential via a combination of static and oscillating electric fields.

The quadrupole mass analyzer, originally conceived by Nobel Laureate Wolfgang Paul and his student Helmut Steinwedel, also known as quadrupole mass filter, is one type of mass analyzer used in mass spectrometry. As the name implies, it consists of four cylindrical rods, set parallel to each other. In a quadrupole mass spectrometer (QMS) the quadrupole is the mass analyzer - the component of the instrument responsible for selecting sample ions based on their mass-to-charge ratio (m/z). Ions are separated in a quadrupole based on the stability of their trajectories in the oscillating electric fields that are applied to the rods.

A quadrupole ion trap or paul trap is a type of ion trap that uses dynamic electric fields to trap charged particles. They are also called radio frequency (RF) traps or Paul traps in honor of Wolfgang Paul, who invented the device and shared the Nobel Prize in Physics in 1989 for this work. It is used as a component of a mass spectrometer or a trapped ion quantum computer.

In mass spectrometry, Orbitrap is an ion trap mass analyzer consisting of an outer barrel-like electrode and a coaxial inner spindle-like electrode that traps ions in an orbital motion around the spindle. The image current from the trapped ions is detected and converted to a mass spectrum using the Fourier transform of the frequency signal.

Electron-transfer dissociation (ETD) is a method of fragmenting multiply-charged gaseous macromolecules in a mass spectrometer between the stages of tandem mass spectrometry (MS/MS). Similar to electron-capture dissociation, ETD induces fragmentation of large, multiply-charged cations by transferring electrons to them. ETD is used extensively with polymers and biological molecules such as proteins and peptides for sequence analysis. Transferring an electron causes peptide backbone cleavage into c- and z-ions while leaving labile post translational modifications (PTM) intact. The technique only works well for higher charge state peptide or polymer ions (z>2). However, relative to collision-induced dissociation (CID), ETD is advantageous for the fragmentation of longer peptides or even entire proteins. This makes the technique important for top-down proteomics. The method was developed by Hunt and coworkers at the University of Virginia.

Time-of-flight mass spectrometry (TOFMS) is a method of mass spectrometry in which an ion's mass-to-charge ratio is determined by a time of flight measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has the same charge. The velocity of the ion depends on the mass-to-charge ratio. The time that it subsequently takes for the ion to reach a detector at a known distance is measured. This time will depend on the velocity of the ion, and therefore is a measure of its mass-to-charge ratio. From this ratio and known experimental parameters, one can identify the ion.

Ion mobility spectrometry–mass spectrometry (IMS-MS) is an analytical chemistry method that separates gas phase ions based on their interaction with a collision gas and their masses. In the first step, the ions are separated according to their mobility through a buffer gas on a millisecond timescale using an ion mobility spectrometer. The separated ions are then introduced into a mass analyzer in a second step where their mass-to-charge ratios can be determined on a microsecond timescale. The effective separation of analytes achieved with this method makes it widely applicable in the analysis of complex samples such as in proteomics and metabolomics.

A triple quadrupole mass spectrometer (TQMS), is a tandem mass spectrometer consisting of two quadrupole mass analyzers in series, with a (non-mass-resolving) radio frequency (RF)–only quadrupole between them to act as a cell for collision-induced dissociation. This configuration is often abbreviated QqQ, here Q1q2Q3.

A reflectron is a type of time-of-flight mass spectrometer that comprises a pulsed ion source, field-free region, ion mirror, and ion detector and uses a static or time dependent electric field in the ion mirror to reverse the direction of travel of the ions entering it. Using the reflectron, one can substantially diminish a spread of flight times of the ions with the same mass-to-charge ratio (m/z) caused by spread in kinetic energy of these ions measured at the exit from the ion source.

A hybrid mass spectrometer is a device for tandem mass spectrometry that consists of a combination of two or more m/z separation devices of different types.
Pittcon Editors’ Awards honoured the best new products on show at the Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, or Pittcon, for 20 years from 1996 having been established by Dr Gordon Wilkinson, managing editor of Analytical Instrument Industry Report. On 8 March 2015, the event returned to the Morial Convention Center in New Orleans and this was the last occasion when the awards were presented.

Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules. In the collision some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry.

A miniature mass spectrometer (MMS) is a type of mass spectrometer (MS) which has small size and weight and can be understood as a portable or handheld device. Current lab-scale mass spectrometers however, usually weigh hundreds of pounds and can cost on the range from thousands to millions of dollars. One purpose of producing MMS is for in situ analysis. This in situ analysis can lead to much simpler mass spectrometer operation such that non-technical personnel like physicians at the bedside, firefighters in a burning factory, food safety inspectors in a warehouse, or airport security at airport checkpoints, etc. can analyze samples themselves saving the time, effort, and cost of having the sample run by a trained MS technician offsite. Although, reducing the size of MS can lead to a poorer performance of the instrument versus current analytical laboratory standards, MMS is designed to maintain sufficient resolutions, detection limits, accuracy, and especially the capability of automatic operation. These features are necessary for the specific in-situ applications of MMS mentioned above.
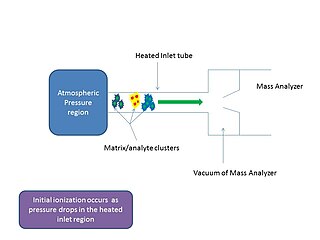
In mass spectrometry, matrix-assisted ionization is a low fragmentation (soft) ionization technique which involves the transfer of particles of the analyte and matrix sample from atmospheric pressure (AP) to the heated inlet tube connecting the AP region to the vacuum of the mass analyzer.

The digital ion trap (DIT) is an quadrupole ion trap driven by digital signals, typically in a rectangular waveform, generated by switching rapidly between discrete DC voltage levels. The digital ion trap has been mainly developed as a mass analyzer.
SCIEX is a manufacturer of mass spectrometry instrumentation used in biomedical and environmental applications. Originally started by scientists from the University of Toronto Institute for Aerospace Studies, it is now part of Danaher Corporation with the SCIEX R&D division still located in Toronto, Canada.
















