
Lipoxygenases are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.

β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.
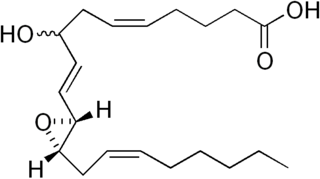
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.
In enzymology, a Delta12-fatty acid dehydrogenase (EC 1.14.99.33) is an enzyme that catalyzes the chemical reaction
In enzymology, a flavone synthase (EC 1.14.11.22) is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonol synthase is an enzyme that catalyzes the following chemical reaction :
In enzymology, a leucocyanidin oxygenase (EC 1.14.11.19) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate 11-lipoxygenase (EC 1.13.11.45) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate diol synthase (EC 1.13.11.44) is an enzyme that catalyzes the chemical reaction

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Epidermis-type lipoxygenase 3 is a member of the lipoxygenase family of enzymes; in humans, it is encoded by the ALOXE3 gene. This gene is located on chromosome 17 at position 13.1 where it forms a cluster with two other lipoxygenases, ALOX12B and ALOX15B. Among the human lipoxygenases, ALOXE3 is most closely related in amino acid sequence to ALOX12B. ALOXE3, ALOX12B, and ALOX15B are often classified as epidermal lipoxygenases, in distinction to the other three human lipoxygenases, because they were initially defined as being highly or even exclusively expressed and functioning in skin. The epidermis-type lipoxygenases are now regarded as a distinct subclass within the multigene family of mammalian lipoxygenases with mouse Aloxe3 being the ortholog to human ALOXE3, mouse Alox12b being the ortholog to human ALOX12B, and mouse Alox8 being the ortholog to human ALOX15B [supplied by OMIM]. ALOX12B and ALOXE3 in humans, Alox12b and Aloxe3 in mice, and comparable orthologs in other in other species are proposed to act sequentially in a multistep metabolic pathway that forms products that are structurally critical for creating and maintaining the skin's water barrier function.
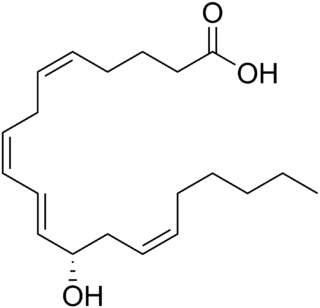
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.
Linoleate 9S-lipoxygenase (EC 1.13.11.58, 9-lipoxygenase, 9S-lipoxygenase, linoleate 9-lipoxygenase, LOX1 (gene), 9S-LOX) is an enzyme with systematic name linoleate:oxygen 9S-oxidoreductase. This enzyme catalyses the following chemical reaction
Linoleate 8R-lipoxygenase (EC 1.13.11.60, linoleic acid 8R-dioxygenase, 5,8-LDS (bifunctional enzyme), 7,8-LDS (bifunctional enzyme), 5,8-linoleate diol synthase (bifunctional enzyme), 7,8-linoleate diol synthase (bifunctional enzyme), PpoA) is an enzyme with systematic name linoleate:oxygen (8R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Linolenate 9R-lipoxygenase (EC 1.13.11.61, NspLOX, (9R)-LOX, linoleate 9R-dioxygenase) is an enzyme with systematic name alpha-linolenate:oxygen (9R)-oxidoreductase. This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8S-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -7,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Functionally, the αKG-dependent hydroxylases are comparable to cytochrome P450 enzymes. Both use O2 and reducing equivalents as cosubstrates and both generate water.

In biochemistry, non-heme iron proteins describe families of enzymes that utilize iron at the active site but lack heme cofactors. Iron-sulfur proteins, including those that are enzymes, are not included in this definition.







