Related Research Articles

The Controlled Substances Act (CSA) is the statute establishing federal U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs.

Propylhexedrine, commonly sold under the brand name Benzedrex, is an alkylamine primarily utilized as a topical nasal decongestant. Its main indications are relief of congestion due to colds, allergies, and allergic rhinitis.

HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 has a binding affinity of 0.061 nM at CB1 and 0.52 nM at CB2 in cloned human cannabinoid receptors compared to delta-9-THC of 40.7 nM at CB1. HU-210 is the (–)-1,1-dimethylheptyl analog of 11-hydroxy- Δ8- tetrahydrocannabinol; in some references it is called 1,1-dimethylheptyl- 11-hydroxytetrahydrocannabinol. The abbreviation "HU" stands for Hebrew University.

In the United States, the removal of cannabis from Schedule I of the Controlled Substances Act is a proposed legal and administrative change in cannabis-related law at the federal level. It has been proposed repeatedly since 1972. The category is the most tightly restricted category reserved for drugs that have "no currently accepted medical use."

Tiletamine is a dissociative anesthetic and pharmacologically classified as an NMDA receptor antagonist. It is related chemically to ketamine. Tiletamine hydrochloride exists as odorless white crystals.

Zolazepam (Flupyrazapon) is a pyrazolodiazepinone derivative structurally related to the benzodiazepine drugs, which is used as an anaesthetic for a wide range of animals in veterinary medicine. Zolazepam is usually administered in combination with other drugs such as the NMDA antagonist tiletamine or the α2 adrenergic receptor agonist xylazine, depending on what purpose it is being used for. It is around four times the potency of diazepam but it is both water-soluble and un-ionized at physiological pH meaning that its onset is very fast.

The drug policy in the United States is the activity of the federal government relating to the regulation of drugs. Starting in the early 1900s, the United States government began enforcing drug policies. These policies criminalized drugs such as opium, morphine, heroin, and cocaine outside of medical use. The drug policies put into place are enforced by the Food and Drug Administration and the Drug Enforcement Administration. Classification of Drugs are defined and enforced using the Controlled Substance Act, which lists different drugs into their respective substances based on its potential of abuse and potential for medical use. Four different categories of drugs are Alcohol, Cannabis, Opioids, and Stimulants.

The Convention on Psychotropic Substances of 1971 is a United Nations treaty designed to control psychoactive drugs such as amphetamine-type stimulants, barbiturates, benzodiazepines, and psychedelics signed in Vienna, Austria on 21 February 1971. The Single Convention on Narcotic Drugs of 1961 did not ban the many newly discovered psychotropics, since its scope was limited to drugs with cannabis, coca and opium-like effects.

Eutylone is a stimulant and empathogenic compound developed in the 1960s, which is classified as a designer drug. It was first reported to the EMCDDA in 2014 and became widespread internationally in 2019-2020 following bans on the related compound ephylone. It is not a natural, but a synthetic cathinone. In 2021, eutylone was the most common cathinone identified by the Drug Enforcement Administration in the United States.

α-Pyrrolidinohexiophenone is a synthetic stimulant drug of the cathinone class developed in the 1960s which has been reported as a novel designer drug.
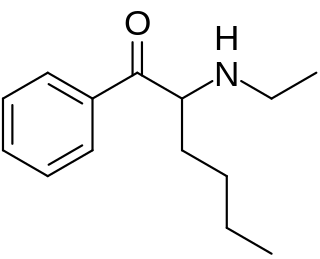
N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.

4-Methylphenethylacetylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug.

5F-MDMB-PICA (MDMB-5F-PICA) is a designer drug and synthetic cannabinoid. In 2018, it was the fifth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

5F-EDMB-PINACA is a designer drug and synthetic cannabinoid. In 2018, it was the fourth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

Serdexmethylphenidate is a prodrug of dexmethylphenidate created by the pharmaceutical company KemPharm. The compound was first approved by the FDA as one of the active ingredients in Azstarys for the treatment of attention deficit hyperactivity disorder (ADHD) in children, adolescents, and adults in March 2021. Serdexmethylphenidate is a prodrug which has a delayed onset of action and a prolonged duration of effects compared to dexmethylphenidate, its parent compound.

Thenylfentanyl is an analogue of fentanyl where the phenethylamine side-chain has been replaced by a thiophenylmethyl group. It was temporarily scheduled by the Drug Enforcement Administration in 1985, due to fears it would be used as a designer drug. But in 2010 the DEA acknowledged it was essentially inactive. Subsequently, the substance was since deregulated.
References
- ↑ "21 CFR 1308.14 (Schedule IV)". Code of Federal Regulations . Retrieved September 9, 2023.
- ↑ retrieved October 2, 2007
- ↑ Bensinger, Peter (August 28, 1978). "Placement of Preparations Containing Difenoxin in Combination With Atropine Sulfate Into Schedules IV and V" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Bensinger, Peter (July 24, 1980). "Classification of Dextropropoxyphene as a Narcotic Drug in Schedule IV of the Controlled Substances Act" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Harrigan, Thomas (July 2, 2014). "Schedules of Controlled Substances: Placement of Tramadol Into Schedule IV". Federal Register. Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Leonhart, Michele (February 27, 2014). "Schedules of Controlled Substances: Placement of Alfaxalone into Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Mullen, Jr., Francis M. (November 12, 1981). "Schedules of Controlled Substsnces; Placement of Alprazolam in Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- 1 2 3 4 5 6 7 8 9 10 11 Bartels Jr., John R. (June 20, 1974). "PART 1308— SCHEDULES OF CONTROLLED SUBSTANCES: Annual Publication" (PDF). Government Publishing Office . Drug Enforcement Administration. Archived from the original (PDF) on September 6, 2023. Retrieved September 6, 2023.
- ↑ Dhillon, Uttam (January 24, 2020). "Schedules of Controlled Substances: Placement of Brexanolone in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved September 6, 2023.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Mullen, Jr., Francis M. (October 5, 1984). "Schedules of Controlled Substances; Temporary Placement of Bromazepam, Camazepam, Clobazam, Clotiazepam, Cloxazolam, Delorazepam, Estazolam, Ethyl Loflazepate, Fludiazepam, Flunitrazepam, Haloxazolam, Ketazolam, Loprazolam, Lormetazepam, Medazepam, Nimetazepam, Nitrazepam, Nordiazepam, Oxazolam, Pinazepam, and Tetrazepam Into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Leonhart, Michele (December 12, 2011). "Schedules of Controlled Substances: Placement of Carisoprodol Into Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- 1 2 3 4 5 6 Bartels, Jr., John R. (May 28, 1975). "Schedules of Controlled Substances; Placement of Chlordiazepoxide, Diazepam, Oxazepam, Chlorazepate, Flurazepam, and Clonazepam into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Milgram, Anne (April 7, 2022). "Schedules of Controlled Substances: Placement of Daridorexant in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Simpkins, William (August 16, 2001). "Schedules of controlled substances: Dichloralphenazone; placement into Schedule IV". VLEX. Drug Enforcement Administration. Archived from the original on January 16, 2023. Retrieved January 16, 2023.
- ↑ Leonhart, Michele (October 6, 2009). "Schedules of Controlled Substances; Placement of Fospropofol Into Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Mullen, Jr., Francis M. (October 29, 1981). "Schedules of Controlled Substances: Placement of Halazepam in Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Evans, D. Christopher (March 3, 2021). "Schedules of Controlled Substances: Placement of Lemborexant in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Bensinger, Peter (October 7, 1977). "Placement of Lorazepam in Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Bartels, Jr., John R. (January 30, 1975). "Placement of Mebutamate in Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2022.
- 1 2 Lawn, John (March 25, 1986). "Schedules of Controlled Substances; Placement of Quazepam and Midazolam into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Bensinger, Peter (December 17, 1976). "Placement of Prazepam into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Evans, D. Christopher (July 2, 2021). "Schedules of Controlled Substances: Placement of Remimazolam in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved September 6, 2023.
- ↑ Harrigan, Thomas (August 28, 2014). "Schedules of Controlled Substances: Placement of Suvorexant into Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Bensinger, Peter (April 7, 1981). "Schedules of Controlled Substances; Placement of Temazepam into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Mullen, Jr., Francis M. (December 28, 1982). "Schedules of Controlled Substances; Placement of Triazolam into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Marshall, Donnie (September 15, 1999). "Schedules of Controlled Substances; Placement of Zaleplon into Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Bonner, Robert (February 5, 1993). "Schedules of Controlled Substances; Placement of Zolpidem into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived from the original (PDF) on March 3, 2022. Retrieved January 16, 2023.
- ↑ Leonhart, Michele (April 4, 2005). "Schedules of Controlled Substances: Placement of Zopiclone Into Schedule IV". Federal Register . Drug Enforcement Administration.
- ↑ Milgram, Anne (October 31, 2023). "Schedules of Controlled Substances: Placement of Zuranolone in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved November 3, 2023.
- ↑ Leonhart, Michele (May 8, 2013). "Schedules of Controlled Substances: Placement of Lorcaserin Into Schedule IV". Federal Register. Drug Enforcement Administration . Retrieved January 16, 2023.
- 1 2 3 4 Lawn, John (May 17, 1988). "Schedules of Controlled Substances; Temporary Placement of Cathine ((+ )-norpseudoephedrine), Fencamfamin, Fenproporex and Mefenorex Into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived from the original (PDF) on March 3, 2022. Retrieved January 16, 2023.
- 1 2 Ingersoll, John (July 6, 1973). "Diethylpropion and Phentermine; Temporary Placement in Schedule IV" (PDF). Isomer Design. Bureau of Narcotics and Dangerous Drugs. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Mullen, Jr., Francis M. (October 27, 1981). "Rescheduling of Mazindol into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on September 26, 2021. Retrieved January 16, 2023.
- ↑ Marshall, Donnie (January 27, 1999). "Schedules of Controlled Substances: Placement of Modafinil Into Schedule IV". Federal Register . Drug Enforcement Administration.
- ↑ Bartels, Jr., John R. (January 28, 1975). "Placement of Pemoline in Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- 1 2 Bensinger, Peter (September 30, 1980). "Pipradrol and SPA in Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Milgram, Anne (July 25, 2022). "Schedules of Controlled Substances: Placement of Serdexmethylphenidate in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved September 6, 2023.
- ↑ Gruden, Peter (February 11, 1998). "Schedules of Controlled Substances: Placement of Sibutramine Into Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Dhillon, Uttam (January 7, 2020). "Schedules of Controlled Substances: Placement of Solriamfetol in Schedule IV". Federal Register . Drug Enforcement Administration . Retrieved January 16, 2023.
- ↑ Bensinger, Peter (February 9, 1979). "Placement of Pentazocine into Schedule IV" (PDF). Isomer Design. Drug Enforcement Administration. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ Milford, Jr., James S. (October 31, 1997). "Schedules of Controlled Substances Placement of Butorphanol Into Schedule IV". Federal Register . Drug Enforcement Administration. Archived from the original on January 16, 2023. Retrieved January 16, 2023.
- ↑ Rosenberg, Chuck (November 12, 2015). "Schedules of Controlled Substances: Placement of Eluxadoline Into Schedule IV". Federal Register . Drug Enforcement Administration. Archived from the original on January 16, 2023. Retrieved January 16, 2023.