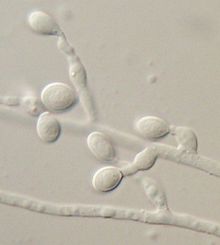
Eumycetoma, also known as Madura foot, is a persistent fungal infection of the skin and the tissues just under the skin, affecting most commonly the feet, although it can occur in hands and other body parts. It starts as a painless wet nodule, which may be present for years before ulceration, swelling, grainy discharge and weeping from sinuses and fistulae, followed by bone deformity.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Cochliobolus lunatus is a fungal plant pathogen that can cause disease in humans and other animals. The anamorph of this fungus is known as Curvularia lunata, while C. lunatus denotes the teleomorph or sexual stage. They are, however, the same biological entity. C. lunatus is the most commonly reported species in clinical cases of reported Cochliobolus infection.
Acremonium strictum is an environmentally widespread saprotroph species found in soil, plant debris, and rotting mushrooms. Isolates have been collected in North and Central America, Asia, Europe and Egypt. A. strictum is an agent of hyalohyphomycosis and has been identified as an increasingly frequent human pathogen in immunosuppressed individuals, causing localized, disseminated and invasive infections. Although extremely rare, A. strictum can infect immunocompetent individuals, as well as neonates. Due to the growing number of infections caused by A. strictum in the past few years, the need for new medical techniques in the identification of the fungus as well as for the treatment of human infections has risen considerably.

Acrophialophora fusispora is a poorly studied ascomycete fungus found in soil, air and various plants. A. fusispora is morphologically similar to the genera Paecilomyces and Masonia, but differ in the presence of pigmented conidiophores, verticillate phialides, and frequent sympodial proliferation. Moreover, A. fusispora is distinguished by its pigmented spindle-shaped conidia, covered with spiral bands. The fungus is naturally found in soils of tropical to temperate regions. The fungus has been identified as a plant and animal pathogen, and has recently been recognized as an emerging opportunistic human pathogen. A. fusispora infection in human is rare and has few documented clinical cases, but due to the rarity of the fungus and potential misidentification, the infections may be underdiagnosed. Clinical cases of A. fusispora include cases of keratitis, pulmonary colonization and infection, and cerebral infections. The fungus also has two documented cases of infection in dogs.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.

Pseudallescheria boydii is a species of fungus classified in the Ascomycota. It is associated with some forms of eumycetoma/maduromycosis and is the causative agent of pseudallescheriasis. Typically found in stagnant and polluted water, it has been implicated in the infection of immunocompromised and near-drowned pneumonia patients. Treatment of infections with P. boydii is complicated by resistance to many of the standard antifungal agents normally used to treat infections by filamentous fungi.
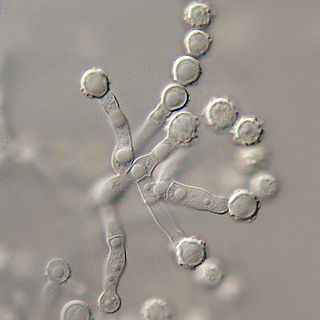
The Microascaceae are a family of fungi in the class Sordariomycetes, subclass Hypocreomycetidae. The family was published by David Malloch in 1970, an emended description based on Everet Stanley Luttrell's original 1951 publication. Family was updated in 2020.

Exophiala dermatitidis is a thermophilic black yeast, and a member of the Herpotrichiellaceae. While the species is only found at low abundance in nature, metabolically active strains are commonly isolated in saunas, steam baths, and dish washers. Exophiala dermatitidis only rarely causes infection in humans, however cases have been reported around the world. In East Asia, the species has caused lethal brain infections in young and otherwise healthy individuals. The fungus has been known to cause cutaneous and subcutaneous phaeohyphomycosis, and as a lung colonist in people with cystic fibrosis in Europe. In 2002, an outbreak of systemic E. dermatitidis infection occurred in women who had received contaminated steroid injections at North Carolina hospitals.
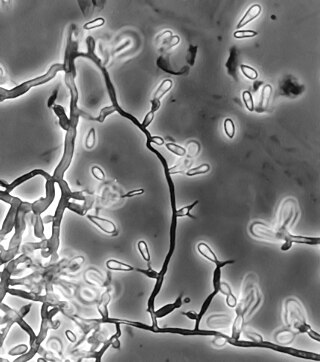
Ochroconis gallopava, also called Dactylaria gallopava or Dactylaria constricta var. gallopava, is a member of genus Dactylaria. Ochroconis gallopava is a thermotolerant, darkly pigmented fungus that causes various infections in fowls, turkeys, poults, and immunocompromised humans first reported in 1986. Since then, the fungus has been increasingly reported as an agent of human disease especially in recipients of solid organ transplants. Ochroconis gallopava infection has a long onset and can involve a variety of body sites. Treatment of infection often involves a combination of antifungal drug therapy and surgical excision.

Apophysomyces variabilis is an emerging fungal pathogen that can cause serious and sometimes fatal infection in humans. This fungus is a soil-dwelling saprobe with tropical to subtropical distribution. It is a zygomycete that causes mucormycosis, an infection in humans brought about by fungi in the order Mucorales. Infectious cases have been reported globally in locations including the Americas, Southeast Asia, India, and Australia. Apophysomyces variabilis infections are not transmissible from person to person.
Phaeohyphomycosis is a diverse group of fungal infections, caused by dematiaceous fungi whose morphologic characteristics in tissue include hyphae, yeast-like cells, or a combination of these. It can be associated with an array of melanistic filamentous fungi including Alternaria species, Exophiala jeanselmei, and Rhinocladiella mackenziei.

Lichtheimia corymbifera is a thermophilic fungus in the phylum Zygomycota. It normally lives as a saprotrophic mold, but can also be an opportunistic pathogen known to cause pulmonary, CNS, rhinocerebral, or cutaneous infections in animals and humans with impaired immunity.
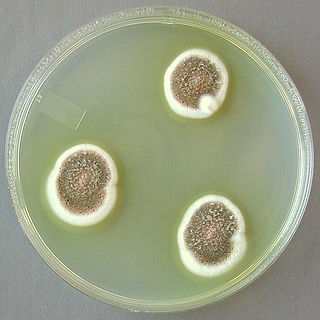
Aspergillus ustus is a microfungus and member of the division Ascomycota. It is commonly found in indoor environments and soil. Isolated cases of human infection resulting from A. ustus have been described; however the majority of these are nail infections.
Scedosporiosis is the general name for any mycosis – i.e., fungal infection – caused by a fungus from the genus Scedosporium. Current population-based studies suggest Scedosporium prolificans and Scedosporium apiospermum to be among the most common infecting agents from the genus, although infections caused by other members thereof are not unheard of. The latter is an asexual form (anamorph) of another fungus, Pseudallescheria boydii. The former is a "black yeast", currently not characterized as well, although both of them have been described as saprophytes.

Scedosporium is a genus of fungi in the family Microascaceae.

Neoscytalidium dimidiatum was first described in 1933 as Hendersonula toruloidea from diseased orchard trees in Egypt. Decades later, it was determined to be a causative agent of human dermatomycosis-like infections and foot infections predominantly in tropical areas; however the fungus is considered to be widespread. A newer name, Scytalidium dimidiatum, was applied to a synanamorph of Nattrassia mangiferae, otherwise known as Neofusicoccum mangiferae. Substantial confusion has arisen in the literature on this fungus resulting from the use of multiple different names including Torula dimidiata, Fusicoccum dimidiatum, Scytalidium dimidiatum, and Hendersonula toruloidea. Additionally, Scytalidium lignicola and Scytalidium lignicolum are often considered earlier names of N. dimidiatum.
Petriella setifera is a fungus commonly found in soil and feces. The fungus has also been located on wood rot, plant species, and compost. A significant portion of P. setifera reports are found on sources with no previous association with the fungus. There are no known human cases of fungal infection, but one reported case of a dolphin infection. The fungus may have immunosuppressive characteristics, but it has not been confirmed. Many properties of the fungus are unknown, requiring further research.

Phialophora verrucosa is a pathogenic, dematiaceous fungus that is a common cause of chromoblastomycosis. It has also been reported to cause subcutaneous phaeohyphomycosis and mycetoma in very rare cases. In the natural environment, it can be found in rotting wood, soil, wasp nests, and plant debris. P. verrucosa is sometimes referred to as Phialophora americana, a closely related environmental species which, along with P. verrucosa, is also categorized in the P. carrionii clade.
Cladophialophora arxii is a black yeast shaped dematiaceous fungus that is able to cause serious phaeohyphomycotic infections. C. arxii was first discovered in 1995 in Germany from a 22-year-old female patient suffering multiple granulomatous tracheal tumours. It is a clinical strain that is typically found in humans and is also capable of acting as an opportunistic fungus of other vertebrates Human cases caused by C. arxii have been reported from all parts of the world such as Germany and Australia.