Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words χλωρός, khloros and φύλλον, phyllon ("leaf"). Chlorophyll allow plants to absorb energy from light.

Magnesium is an essential element in biological systems. Magnesium occurs typically as the Mg2+ ion. It is an essential mineral nutrient (i.e., element) for life and is present in every cell type in every organism. For example, adenosine triphosphate (ATP), the main source of energy in cells, must bind to a magnesium ion in order to be biologically active. What is called ATP is often actually Mg-ATP. As such, magnesium plays a role in the stability of all polyphosphate compounds in the cells, including those associated with the synthesis of DNA and RNA.
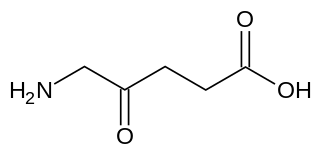
δ-Aminolevulinic acid, an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in plants.
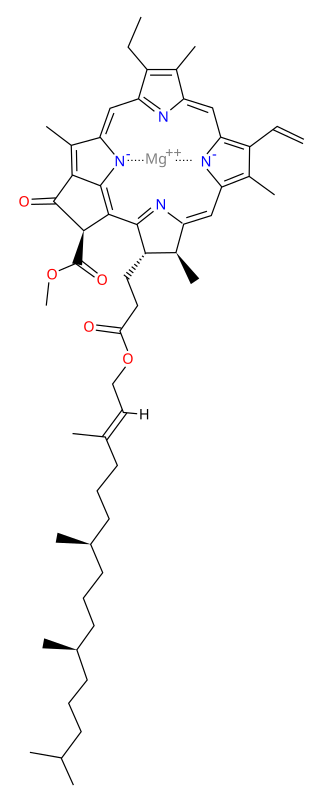
Chlorophyll a is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll a also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located.

Chlorophyll b is a form of chlorophyll. Chlorophyll b helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll a in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light.

Protoporphyrin ferrochelatase (EC 4.98.1.1, formerly EC 4.99.1.1, or ferrochelatase; systematic name protoheme ferro-lyase (protoporphyrin-forming)) is an enzyme encoded by the FECH gene in humans. Ferrochelatase catalyses the eighth and terminal step in the biosynthesis of heme, converting protoporphyrin IX into heme B. It catalyses the reaction:

Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not soluble in water. The name is often abbreviated as PPIX.

Ribose-phosphate diphosphokinase is an enzyme that converts ribose 5-phosphate into phosphoribosyl pyrophosphate (PRPP). It is classified under EC 2.7.6.1.

In enzymology, a magnesium protoporphyrin IX methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, divinyl chlorophyllide a 8-vinyl-reductase (EC 1.3.1.75) is an enzyme that catalyzes the chemical reaction
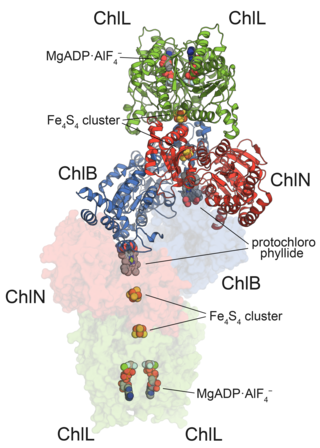
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide a. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.
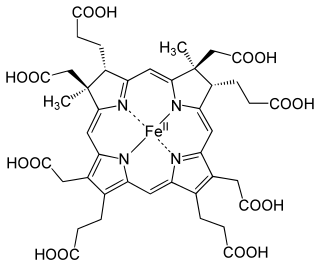
The enzyme sirohydrochlorin ferrochelatase (EC 4.99.1.4) catalyzes the following reaction:

Cobalt chelatase (EC 6.6.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a Mg2+-importing ATPase (EC 3.6.3.2) is an enzyme that catalyzes the chemical reaction

In enzymology, chlorophyll synthase is an enzyme that catalyzes the chemical reaction

Chlorophyllide-a oxygenase (EC 1.14.13.122), chlorophyllide a oxygenase, chlorophyll-b synthase, CAO) is an enzyme with systematic name chlorophyllide-a:oxygen 7-oxidoreductase. This enzyme catalyses the following chemical reactions

Magnesium-protoporphyrin IX monomethyl ester (oxidative) cyclase, is an enzyme with systematic name magnesium-protoporphyrin-IX 13-monomethyl ester, ferredoxin:oxygen oxidoreductase (hydroxylating). In plants this enzyme catalyses the following overall chemical reaction

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.
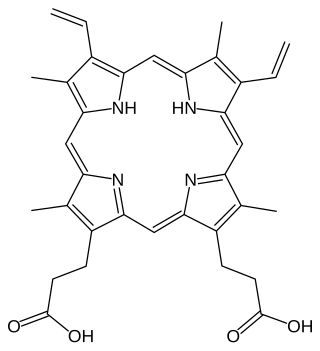
In biochemistry, chelatases are enzymes that catalyze the insertion ("metalation") of naturally occurring tetrapyrroles. Many tetrapyrrole-based cofactors exist in nature including hemes, chlorophylls, and vitamin B12. These metallo cofactors are derived by the reaction of metal cations with tetrapyrroles, which are not ligands per se, but the conjugate acids thereof. In the case of ferrochelatases, the reaction that chelatases catalyze is:

Chlorophyllide a reductase (EC 1.3.7.15), also known as COR, is an enzyme with systematic name bacteriochlorophyllide-a:ferredoxin 7,8-oxidoreductase. It catalyses the following chemical reaction