 | |
| Names | |
|---|---|
| Preferred IUPAC name Methyl dimethylcarbamodithioate | |
| Other names Cystogon, DMDTM, Forbiat | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.021.005 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
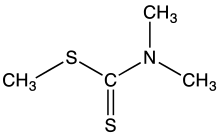
| (CH3)2NC(S)SCH3 | |
| Molar mass | 135.24 g·mol−1 |
| Appearance | colorless or white solid |
| Melting point | 45–47 °C (113–117 °F; 318–320 K) |
| Hazards | |
| GHS labelling: [1] | |
 | |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Methyl dimethyldithiocarbamate is the organosulfur compound with the formula (CH3)2NC(S)SCH3. It is the one of simplest dithiocarbamic esters. It is a white volatile solid that is poorly soluble in water but soluble in many organic solvents. It was once used as a pesticide.
Methyl dimethyldithiocarbamate can be prepared by methylation of salts of dimethyldithiocarbamate: [2]
- (CH3)2NCS−2Na+ + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + CH3OSO−3Na+
It can also be prepared by the reaction of a tetramethylthiuram disulfide with methyl Grignard reagents: [3]
- [(CH3)2NC(S)S]2 + CH3MgBr → (CH3)2NC(S)SCH3 + (CH3)2NCS2MgBr