
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.
Dimethylformamide is an organic compound with the formula (CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions.
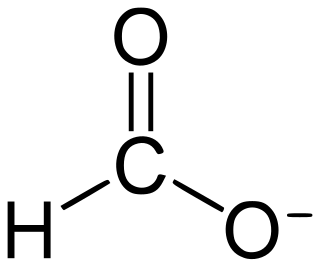
Formate is the conjugate base of formic acid. Formate is an anion or its derivatives such as ester of formic acid. The salts and esters are generally colorless.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
In enzymology, a 2-hydroxymuconate-semialdehyde hydrolase (EC 3.7.1.9) is an enzyme that catalyzes the chemical reaction
The enzyme formyl-CoA hydrolase (EC 3.1.2.10) catalyzes the reaction
The enzyme S-formylglutathione hydrolase (EC 3.1.2.12) catalyzes the reaction
In enzymology, an arylformamidase (EC 3.5.1.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a formamidase (EC 3.5.1.49) is an enzyme that catalyzes the chemical reaction
In enzymology, a formylaspartate deformylase (EC 3.5.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, a formylmethionine deformylase (EC 3.5.1.31) is an enzyme that catalyzes the chemical reaction
In enzymology, a formyltetrahydrofolate deformylase (EC 3.5.1.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a GTP cyclohydrolase II (EC 3.5.4.25) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-formylglutamate deformylase (EC 3.5.1.68) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-formylmethionylaminoacyl-tRNA deformylase (EC 3.5.1.27) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-substituted formamide deformylase (EC 3.5.1.91) is an enzyme that catalyzes the chemical reaction
In enzymology, a succinylglutamate desuccinylase (EC 3.5.1.96) is an enzyme that catalyzes the chemical reaction
In chemistry, decarbonylation is a type of organic reaction that involves the loss of carbon monoxide (CO). It is often an undesirable reaction, since it represents a degradation. In the chemistry of metal carbonyls, decarbonylation describes a substitution process, whereby a CO ligand is replaced by another ligand.

tert-Butoxybis(dimethylamino)methane is an organic compound with the formula (CH3)3COCH(N(CH3)2)2. The compound is classified as an aminal ester, i.e. the tert-butyl alcohol derivative of the aminal bis(dimethylamino)methane. It is a colorless liquid with a amine odor.





