Aromatic compounds are those chemical compounds that contain one or more rings with pi electrons delocalized all the way around them. In contrast to compounds that exhibit aromaticity, aliphatic compounds lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered, and referred simply to the fact that many such compounds have a sweet or pleasant odour; however, not all aromatic compounds have a sweet odour, and not all compounds with a sweet odour are aromatic. Aromatic hydrocarbons, or arenes, are aromatic organic compounds containing solely carbon and hydrogen atoms. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simple aromatic compound benzene, or a phenyl group when part of a larger compound.

In organic chemistry, hydrocarbons are divided into two classes: aromatic compounds and aliphatic compounds, also known as non-aromatic hydrocarbons. Aliphatics can be cyclic; however, hydrocarbons with conjugated pi-systems that obey Hückel's rule are instead considered to demonstrate aromaticity. Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds contain no rings of any type, and are thus aliphatic.
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles.
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates. Antoine Lavoisier suggested instead the name azote, from the Greek ἀζωτικός "no life", as it is an asphyxiant gas; this name is used instead in many languages, such as French, Italian, Russian, Romanian, Portuguese and Turkish, and appears in the English names of some nitrogen compounds such as hydrazine, azides and azo compounds.

In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen bonds. Due to carbon's ability to catenate, millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprises the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds, along with a handful of other exceptions, are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive.

Organic chemistry is a branch of chemistry that studies the structure, properties and reactions of organic compounds, which contain carbon in covalent bonding. Study of structure determines their chemical composition and formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.

In chemistry, an oxidizing agent, or oxidising agent (oxidiser) is a substance that has the ability to oxidize other substances — in other words to accept their electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. However, the distinction is not clearly defined; authorities have differing views on the subject. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.
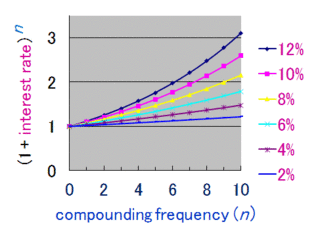
Compound interest is the addition of interest to the principal sum of a loan or deposit, or in other words, interest on interest. It is the result of reinvesting interest, rather than paying it out, so that interest in the next period is then earned on the principal sum plus previously accumulated interest. Compound interest is standard in finance and economics.

Azo compounds are compounds bearing the functional group diazenyl R−N=N−R′, in which R and R′ can be either aryl or alkyl.

Substituted methylenedioxy- phenethylamines (MDxx) are a large chemical class of derivatives of the phenethylamines, which includes many psychoactive drugs that act as entactogens, psychedelics, and/or stimulants, as well as entheogens. These agents are used as research chemicals, designer drugs and as recreational substances.

Substituted phenethylamines are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents.
Drugs controlled by the United Kingdom (UK) Misuse of Drugs Act 1971 are listed in this article. These drugs are known in the UK as controlled drugs, because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and other substances which may be considered drugs are controlled by other laws.
The molecular formula C4H8N2O2 may refer to:













