Hydrolase is a class of enzymes that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases.
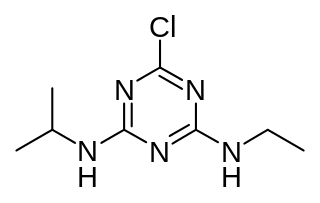
Atrazine is a chlorinated herbicide of the triazine class. It is used to prevent pre-emergence broadleaf weeds in crops such as maize (corn), soybean and sugarcane and on turf, such as golf courses and residential lawns. Atrazine's primary manufacturer is Syngenta and it is one of the most widely used herbicides in the United States, Canadian, and Australian agriculture. Its use was banned in the European Union in 2004, when the EU found groundwater levels exceeding the limits set by regulators, and Syngenta could not show that this could be prevented nor that these levels were safe.
Atrazine Chlorohydrolase (AtzA) is an enzyme (E.C.3.8.1.8), which catalyzes the conversion of atrazine to hydroxyatrazine. Bacterial degradation determines the environmental impact and efficacy of an herbicide or pesticide. Initially, most pesticides are highly effective and show minimal bacterial degradation; however, bacteria can rapidly evolve and gain the ability to metabolize potential nutrients in the environment. Despite a remarkable structural similarity, degradation of atrazine by bacteria capable of melamine degradation was rare; however, since its introduction as a pesticide in the United States, bacteria capable of atrazine degradation have evolved. Currently, Pseudomonas sp. strain ADP seems to be the optimal bacterial strain for atrazine degradations, which appears to be the sole nitrogen source for the bacteria.
In enzymology, 2-aminomuconate deaminase (EC 3.5.99.5) (also known as amnd) is an enzyme that catalyzes the chemical reaction
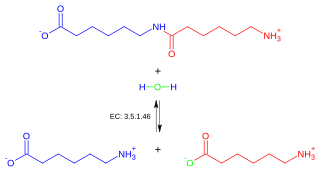
In enzymology, a 6-aminohexanoate-dimer hydrolase (EC 3.5.1.46) is an enzyme that catalyzes the chemical reaction N-(6-aminohexanoyl)-6-aminohexanoate + H2O 2 6-aminohexanoate. Thus, the two substrates of this enzyme are N-(6-aminohexanoyl)-6-aminohexanoate and H2O, whereas its product is two molecules of 6-aminohexanoate.
In enzymology, an adenosine-phosphate deaminase (EC 3.5.4.17) is an enzyme that catalyzes the chemical reaction
In enzymology, an allophanate hydrolase (EC 3.5.1.54) is an enzyme that catalyzes the chemical reaction
In enzymology, a beta-ureidopropionase (EC 3.5.1.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a biuret amidohydrolase (EC 3.5.1.84) is an enzyme that catalyzes the chemical reaction
In enzymology, a cyanuric acid amidohydrolase (EC 3.5.2.15) is an enzyme that catalyzes the chemical reaction
In enzymology, a formimidoylaspartate deiminase (EC 3.5.3.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a formylaspartate deformylase (EC 3.5.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, a hydroxydechloroatrazine ethylaminohydrolase (EC 3.5.99.3) is an enzyme that catalyzes the chemical reaction
In enzymology, an imidazolonepropionase (EC 3.5.2.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetyldiaminopimelate deacetylase (EC 3.5.1.47) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acyl-D-amino-acid deacylase (EC 3.5.1.81) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-carbamoyl-D-amino acid hydrolase (EC 3.5.1.77) is an enzyme that catalyzes the chemical reaction
In enzymology, a succinyl-diaminopimelate desuccinylase (EC 3.5.1.18) is an enzyme that catalyzes the chemical reaction
In enzymology, a succinylglutamate desuccinylase (EC 3.5.1.96) is an enzyme that catalyzes the chemical reaction
Pesticide degradation is the process by which a pesticide is transformed into a benign substance that is environmentally compatible with the site to which it was applied. Globally, an estimated 1 to 2.5 million tons of active pesticide ingredients are used each year, mainly in agriculture. Forty percent are herbicides, followed by insecticides and fungicides. Since their initial development in the 1940s, multiple chemical pesticides with different uses and modes of action have been employed. Pesticides are applied over large areas in agriculture and urban settings. Pesticide use, therefore, represents an important source of diffuse chemical environmental inputs.




