The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone, however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

Copper(II) chloride is the chemical compound with the chemical formula CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

1,2-Naphthoquinone or ortho-naphthoquinone is a polycyclic aromatic organic compound with formula C
10H
6O
2. This yellow solid is prepared by oxidation of 1-amino-2-hydroxynaphthalene with ferric chloride.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.

Juglone, also called 5-hydroxy-1,4-naphthalenedione (IUPAC) is an organic compound with the molecular formula C10H6O3. In the food industry, juglone is also known as C.I. Natural Brown 7 and C.I. 75500. It is insoluble in benzene but soluble in dioxane, from which it crystallizes as yellow needles. It is an isomer of lawsone, which is the staining compound in the henna leaf.
In enzymology, juglone 3-monooxygenase (EC 1.14.99.27) is an enzyme that catalyzes the chemical reaction
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.

2,3,5,6,7,8-Hexahydroxy-1,4-naphthalenedione, also called hexahydroxynaphthoquinone or spinochrome E, is an organic compound with formula C
10H
6O
8. It is formally derived from naphthoquinone (1,4-naphtalenedione) through replacement of all six hydrogen atoms by hydroxyl (OH) groups. The numerical prefixes "2,3,5,6,7,8" are superfluous, since there is no other hexahydroxy derivative of 1,4-naphthoquinone.
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
A dihydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of naphthoquinone through replacement of two hydrogen atoms (H) by hydroxyl groups (OH).
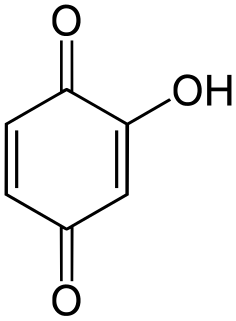
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone.
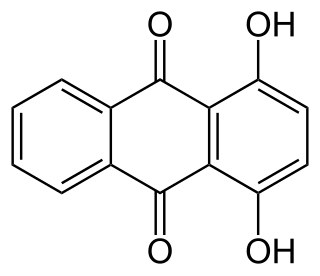
A hydroxyanthraquinone (formula: C14H9O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

1,4-Naphthoquinone or para-naphthoquinone is an organic compound derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.
1,4-dihydroxy-2-naphthoyl-CoA hydrolase (EC 3.1.2.28) is an enzyme with systematic name 1,4-dihydroxy-2-naphthoyl-CoA hydrolase. This enzyme catalyses the following chemical reaction

1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.

Homoisoflavonoids (3-benzylidenechroman-4-ones) are a type of phenolic compounds occurring naturally in plants.