Related Research Articles

Lymphoma is a group of blood and lymph tumors that develop from lymphocytes. The name typically refers to just the cancerous versions rather than all such tumours. Signs and symptoms may include enlarged lymph nodes, fever, drenching sweats, unintended weight loss, itching, and constantly feeling tired. The enlarged lymph nodes are usually painless. The sweats are most common at night.
Post-transplant lymphoproliferative disorder (PTLD) is the name given to a B cell proliferation due to therapeutic immunosuppression after organ transplantation. These patients may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B cells may undergo mutations which will render them malignant, giving rise to a lymphoma.

Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.
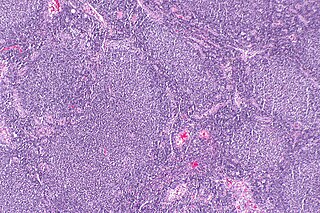
Follicular lymphoma (FL) is a cancer that involves certain types of white blood cells known as lymphocytes. The cancer originates from the uncontrolled division of specific types of B-cells known as centrocytes and centroblasts. These cells normally occupy the follicles (nodular swirls of various types of lymphocytes) in the germinal centers of lymphoid tissues such as lymph nodes. The cancerous cells in FL typically form follicular or follicle-like structures (see adjacent Figure) in the tissues they invade. These structures are usually the dominant histological feature of this cancer.
Enteropathy refers to any pathology of the intestine. Although enteritis specifically refers to an inflammation of the intestine, and is thus a more specific term than "enteropathy", the two phrases are sometimes used interchangeably.
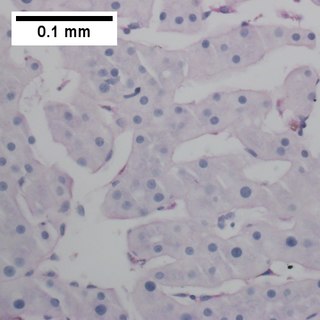
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.

T-cell lymphoma is a rare form of cancerous lymphoma affecting T-cells. Lymphoma arises mainly from the uncontrolled proliferation of T-cells and can become cancerous.
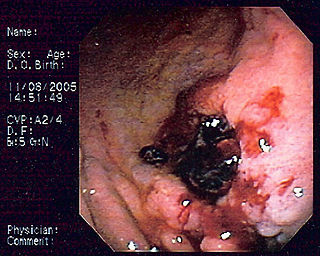
Primary gastric lymphoma is an uncommon condition, accounting for less than 15% of gastric malignancies and about 2% of all lymphomas. However, the stomach is a very common extranodal site for lymphomas. It is also the most common source of lymphomas in the gastrointestinal tract.

Intravascular lymphomas (IVL) are rare cancers in which malignant lymphocytes proliferate and accumulate within blood vessels. Almost all other tyes of lymphoma involve the proliferation and accumulation of malignant lymphocytes in lymph nodes, other parts of the lymphatic system, and various non-lymphatic organs but not in blood vessels.
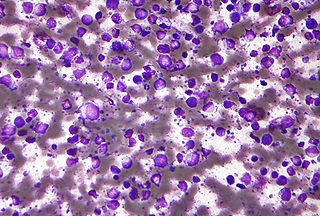
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.
Lymphomatoid granulomatosis (LYG or LG) is a very rare lymphoproliferative disorder first characterized in 1972. Lymphomatoid means lymphoma-like and granulomatosis denotes the microscopic characteristic of the presence of granulomas with polymorphic lymphoid infiltrates and focal necrosis within it.

Enteropathy-associated T-cell lymphoma (EATL), previously termed enteropathy-associated T-cell lymphoma, type I and at one time termed enteropathy-type T-cell lymphoma (ETTL), is a complication of coeliac disease in which a malignant T-cell lymphoma develops in areas of the small intestine affected by the disease's intense inflammation. While a relatively rare disease, it is the most common type of primary gastrointestinal T-cell lymphoma.

Marginal zone B-cell lymphomas, also known as marginal zone lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue, the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body such as the gastrointestinal tract, mouth, nasal cavity, pharynx, thyroid gland, breast, lung, salivary glands, eye, skin and the human spleen.

Extranodal NK/T-cell lymphoma, nasal type (ENKTCL-NT) is a rare type of lymphoma that commonly involves midline areas of the nasal cavity, oral cavity, and/or pharynx At these sites, the disease often takes the form of massive, necrotic, and extremely disfiguring lesions. However, ENKTCL-NT can also involve the eye, larynx, lung, gastrointestinal tract, skin, and various other tissues. ENKTCL-NT mainly affects adults; it is relatively common in Asia and to lesser extents Mexico, Central America, and South America but is rare in Europe and North America. In Korea, ENKTCL-NT often involves the skin and is reported to be the most common form of cutaneous lymphoma after mycosis fungoides.
Lethal midline granuloma (LMG) is an historical term for a condition in which necrotic and highly destructive lesions develop progressively in the middle of the face, principally the nose and palate. Many cases presented with ulcerations in or perforations of the palate.
Epstein–Barr virus–associated lymphoproliferative diseases are a group of disorders in which one or more types of lymphoid cells, i.e. B cells, T cells, NK cells, and histiocytic-dendritic cells, are infected with the Epstein–Barr virus (EBV). This causes the infected cells to divide excessively, and is associated with the development of various non-cancerous, pre-cancerous, and cancerous lymphoproliferative disorders (LPDs). These LPDs include the well-known disorder occurring during the initial infection with the EBV, infectious mononucleosis, and the large number of subsequent disorders that may occur thereafter. The virus is usually involved in the development and/or progression of these LPDs although in some cases it may be an "innocent" bystander, i.e. present in, but not contributing to, the disease.
Indolent T cell lymphoproliferative disorder of the gastrointestinal tract or Indolent T cell lymphoproliferative disorder of the GI tract (ITCLD-GT) is a rare and recently recognized disorder in which mature T cell lymphocytes accumulation abnormally in the gastrointestinal tract. This accumulation causes various lesions in the mucosal layer lining the GI tract. Individuals with ITCLD-GT commonly complain of chronic GI tract symptoms such as nausea, vomiting, diarrhea, abdominal pain, and rectal bleeding.
Monomorphic epitheliotropic intestinal T cell lymphoma (MEITL) is an extremely rare peripheral T-cell lymphoma that involves the malignant proliferation of a type of lymphocyte, the T cell, in the gastrointestinal tract. Over time, these T cells commonly spread throughout the mucosal lining of a portion of the GI tract, lead to GI tract nodules and ulcerations, and cause symptoms such as abdominal pain, weight loss, diarrhea, obstruction, bleeding, and/or perforation.
Duodenal-type follicular lymphoma (DFL) is a form of lymphoma in which certain lymphocyte types, the B-cell-derived centrocytes and centroblasts, form lymph node follicle-like structures principally in the duodenum and other parts of the small intestine. It is an indolent disease which on rare occasions progresses to a more aggressive lymphoma that spreads beyond these originally involved sites.
Helicobacter heilmannii sensu lato refers to a group of bacterial species within the Helicobacter genus. The Helicobacter genus consists of at least 40 species of spiral-shaped flagellated, Gram-negative bacteria of which the by far most prominent and well-known species is Helicobacter pylori. H. pylori is associated with the development of gastrointestinal tract diseases such as stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers that are not lymphomas, and various subtypes of extranodal marginal zone lymphomas, e.g. those of the stomach, small intestines, large intestines, and rectumn. H. pylori has also been associated with the development of bile duct cancer and has been associated with a wide range of other diseases although its role in the development of many of these other diseases requires further study.
References
- ↑ O'Connor OA, Bhagat G, Ganapathi K, Pedersen MB, D'Amore F, Radeski D, Bates SE (October 2014). "Changing the paradigms of treatment in peripheral T-cell lymphoma: from biology to clinical practice". Clinical Cancer Research. 20 (20): 5240–54. doi: 10.1158/1078-0432.CCR-14-2020 . PMID 25320373.
- 1 2 3 4 5 Skinnider BF (January 2018). "Lymphoproliferative Disorders of the Gastrointestinal Tract". Archives of Pathology & Laboratory Medicine. 142 (1): 44–52. doi: 10.5858/arpa.2016-0610-RA . PMID 28829152.
- ↑ Vega F, Chang CC, Schwartz MR, Preti HA, Younes M, Ewton A, Verm R, Jaffe ES (April 2006). "Atypical NK-cell proliferation of the gastrointestinal tract in a patient with antigliadin antibodies but not celiac disease". The American Journal of Surgical Pathology. 30 (4): 539–44. doi:10.1097/00000478-200604000-00017. PMID 16625103. S2CID 29115000.
- ↑ Takeuchi K, Yokoyama M, Ishizawa S, Terui Y, Nomura K, Marutsuka K, Nunomura M, Fukushima N, Yagyuu T, Nakamine H, Akiyama F, Hoshi K, Matsue K, Hatake K, Oshimi K (December 2010). "Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation". Blood. 116 (25): 5631–7. doi: 10.1182/blood-2010-06-290650 . PMID 20829373.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Xia D, Morgan EA, Berger D, Pinkus GS, Ferry JA, Zukerberg LR (January 2019). "NK-Cell Enteropathy and Similar Indolent Lymphoproliferative Disorders: A Case Series With Literature Review". American Journal of Clinical Pathology. 151 (1): 75–85. doi: 10.1093/ajcp/aqy108 . PMID 30212873. S2CID 52272766.
- 1 2 3 4 5 6 Matnani R, Ganapathi KA, Lewis SK, Green PH, Alobeid B, Bhagat G (March 2017). "Indolent T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: a review and update". Hematological Oncology. 35 (1): 3–16. doi: 10.1002/hon.2317 . PMID 27353398. S2CID 21364706.
- 1 2 3 4 5 6 Ganapathi KA, Pittaluga S, Odejide OO, Freedman AS, Jaffe ES (September 2014). "Early lymphoid lesions: conceptual, diagnostic and clinical challenges". Haematologica. 99 (9): 1421–32. doi:10.3324/haematol.2014.107938. PMC 4562530 . PMID 25176983.
- 1 2 Takata K, Noujima-Harada M, Miyata-Takata T, Ichimura K, Sato Y, Miyata T, Naruse K, Iwamoto T, Tari A, Masunari T, Sonobe H, Okada H, Iwamuro M, Mizobuchi K, Gion Y, Yoshino T (September 2015). "Clinicopathologic analysis of 6 lymphomatoid gastropathy cases: expanding the disease spectrum to CD4-CD8+ cases". The American Journal of Surgical Pathology. 39 (9): 1259–66. doi:10.1097/PAS.0000000000000443. PMID 25929350. S2CID 32888449.
- 1 2 3 4 5 6 7 8 9 Foukas PG, de Leval L (January 2015). "Recent advances in intestinal lymphomas". Histopathology. 66 (1): 112–36. doi:10.1111/his.12596. PMID 25639480. S2CID 20669863.
- 1 2 3 Isom JA, Arroyo MR, Reddy D, Joshi-Guske P, Al-Quran SZ, Li Y, Allan RW (August 2018). "NK cell enteropathy: a case report with 10 years of indolent clinical behaviour". Histopathology. 73 (2): 345–350. doi:10.1111/his.13502. PMID 29474745. S2CID 3540677.
- 1 2 3 4 Weindorf SC, Smith LB, Owens SR (November 2018). "Update on Gastrointestinal Lymphomas". Archives of Pathology & Laboratory Medicine. 142 (11): 1347–1351. doi: 10.5858/arpa.2018-0275-RA . PMID 30407861.
- ↑ Chander U, Leeman-Neill RJ, Bhagat G (August 2018). "Pathogenesis of Enteropathy-Associated T Cell Lymphoma". Current Hematologic Malignancy Reports. 13 (4): 308–317. doi:10.1007/s11899-018-0459-5. PMID 29943210. S2CID 49430640.
- 1 2 Terai T, Sugimoto M, Uozaki H, Kitagawa T, Kinoshita M, Baba S, Yamada T, Osawa S, Sugimoto K (May 2012). "Lymphomatoidgastropathy mimicking extranodal NK/T cell lymphoma, nasal type: a case report". World Journal of Gastroenterology. 18 (17): 2140–4. doi:10.3748/wjg.v18.i17.2140. PMC 3342615 . PMID 22563204.