
Tularemia, also known as rabbit fever, is an infectious disease caused by the bacterium Francisella tularensis. Symptoms may include fever, skin ulcers, and enlarged lymph nodes. Occasionally, a form that results in pneumonia or a throat infection may occur.
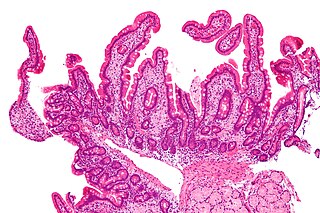
Whipple's disease is a rare systemic infectious disease caused by the bacterium Tropheryma whipplei. First described by George Hoyt Whipple in 1907 and commonly considered as a gastrointestinal disorder, Whipple's disease primarily causes malabsorption, but may affect any part of the human body, including the heart, brain, joints, skin, lungs and the eyes. Weight loss, diarrhea, joint pain, and arthritis are common presenting symptoms, but the presentation can be highly variable in certain individuals, and about 15% of patients do not have the standard signs and symptoms.

Rickettsia rickettsii is a Gram-negative, intracellular, cocco-bacillus bacterium that was first discovered in 1902. Having a reduced genome, the bacterium harvests nutrients from its host cell to carry out respiration, making it an organo-heterotroph. Maintenance of its genome is carried out through vertical gene transfer where specialization of the bacterium allows it to shuttle host sugars directly into its TCA cycle.

Carrion's disease is an infectious disease produced by Bartonella bacilliformis infection.
Rat-bite fever (RBF) is an acute, febrile human illness caused by bacteria transmitted by rodents, in most cases, which is passed from rodent to human by the rodent's urine or mucous secretions. Alternative names for rat-bite fever include streptobacillary fever, streptobacillosis, spirillary fever, bogger, and epidemic arthritic erythema. It is a rare disease spread by infected rodents and caused by two specific types of bacteria:
- Streptobacillus moniliformis, the only reported bacteria that causes RBF in North America
- Spirillum minus, common in Asia. Most cases occur in Japan, but specific strains of the disease are present in the United States, Europe, Australia, and Africa.

Strangles is a contagious upper respiratory tract infection of horses and other equines caused by a Gram-positive bacterium, Streptococcus equi. As a result, the lymph nodes swell, compressing the pharynx, larynx, and trachea, and can cause airway obstruction leading to death, hence the name strangles. Strangles is enzootic in domesticated horses worldwide. The contagious nature of the infection has at times led to limitations on sporting events.

Lymphoma (lymphosarcoma) in animals is a type of cancer defined by a proliferation of malignant lymphocytes within solid organs such as the lymph nodes, bone marrow, liver and spleen. The disease also may occur in the eye, skin, and gastrointestinal tract.

Anaplasma phagocytophilum is a Gram-negative bacterium that is unusual in its tropism to neutrophils. It causes anaplasmosis in sheep and cattle, also known as tick-borne fever and pasture fever, and also causes the zoonotic disease human granulocytic anaplasmosis.

Ehrlichiosis is a tick-borne bacterial infection, caused by bacteria of the family Anaplasmataceae, genera Ehrlichia and Anaplasma. These obligate intracellular bacteria infect and kill white blood cells.
Potomac Horse Fever (PHF) is a potentially-fatal febrile illness affecting horses caused by the intracellular bacterium Neorickettsia risticii. PHF is also known as Shasta River Crud and Equine Monocytic Ehrlichiosis. It was first described in areas surrounding the Potomac River northwest of Washington, D.C., in the 1980s, but cases have been described in many other parts of the United States, such as Minnesota, California, and Pennsylvania. Currently, it is found in more than 40 U.S. states and Canada.
Neorickettsia is a genus of bacteria. Species or strains in this genus are coccoid or pleomorphic cells that reside in cytoplasmic vacuoles within monocytes and macrophages of dogs, horses, bats, and humans.

Human granulocytic anaplasmosis (HGA) is a tick-borne, infectious disease caused by Anaplasma phagocytophilum, an obligate intracellular bacterium that is typically transmitted to humans by ticks of the Ixodes ricinus species complex, including Ixodes scapularis and Ixodes pacificus in North America. These ticks also transmit Lyme disease and other tick-borne diseases.
Rickettsia typhi is a small, aerobic, obligate intracellular, rod shaped gram negative bacterium. It belongs to the typhus group of the Rickettsia genus, along with R. prowazekii. R. typhi has an uncertain history, as it may have long gone shadowed by epidemic typhus. This bacterium is recognized as a biocontainment level 2/3 organism. R. typhi is a flea-borne disease that is best known to be the causative agent for the disease murine typhus, which is an endemic typhus in humans that is distributed worldwide. As with all rickettsial organisms, R. typhi is a zoonotic agent that causes the disease murine typhus, displaying non-specific mild symptoms of fevers, headaches, pains and rashes. There are two cycles of R. typhi transmission from animal reservoirs containing R. typhi to humans: a classic rat-flea-rat cycle that is most well studied and common, and a secondary periodomestic cycle that could involve cats, dogs, opossums, sheep, and their fleas.
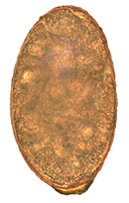
Nanophyetus salmincola is a food-borne intestinal trematode parasite prevalent on the Pacific Northwest coast. The species may be the most common trematode endemic to the United States.

Juga is a genus of freshwater snails with a gill and an operculum, aquatic gastropod mollusks in the family Semisulcospiridae.

African tick bite fever (ATBF) is a bacterial infection spread by the bite of a tick. Symptoms may include fever, headache, muscle pain, and a rash. At the site of the bite there is typically a red skin sore with a dark center. The onset of symptoms usually occurs 4–10 days after the bite. Complications are rare but may include joint inflammation. Some people do not develop symptoms.

Cat-scratch disease (CSD) is an infectious disease that most often results from a scratch or bite of a cat. Symptoms typically include a non-painful bump or blister at the site of injury and painful and swollen lymph nodes. People may feel tired, have a headache, or a fever. Symptoms typically begin within 3–14 days following infection.
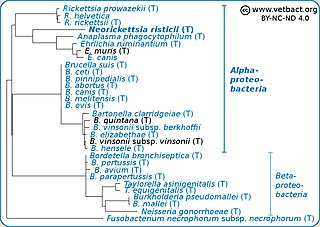
Neorickettsia risticii, formerly Ehrlichia risticii, is an obligate intracellular gram negative bacteria that typically lives as an endosymbiont in parasitic flatworms, specifically flukes. N. risticii is the known causative agent of equine neorickettsiosis, which gets its name from its discovery near the Potomac River in Maryland and Virginia. N. risticii was first recovered from horses in this region in 1984 but was not recognized as the causative agent of PHF until 1979. Potomac horse fever is currently endemic in the United States but has also been reported with lower frequency in other regions, including Canada, Brazil, Uruguay, and Europe. PHF is a condition that is clinically important for horses since it can cause serious signs such as fever, diarrhea, colic, and laminitis. PHF has a fatality rate of approximately 30%, making this condition one of the concerns for horse owners in endemic regions N. risticii is typically acquired in the middle to late summer near freshwater streams or rivers, as well as on irrigated pastures. This is a seasonal infection because it relies on the ingestion of an arthropod vector, which is more commonly found on pasture in the summer months. Although N. risticii is a well known causative agent for PHF in horses, it may act as a potential pathogen in cats and dogs as well. Not only has N. risticii been successfully cultured from monocytes of dogs and cats, but cats have become clinically ill after experimental infection with the bacteria. In addition, N. risticii has been isolated and cultured from human histiocytic lymphoma cells.
Amphistomiasis is a parasitic disease of livestock animals, more commonly of cattle and sheep, and humans caused by immature helminthic flatworms belonging to the order Echinostomida. The term amphistomiasis is used for broader connotation implying the disease inflicted by members of Echinostomida including the family Paramphistomidae/Gastrodiscidae ; whereas paramphistomiasis is restricted to that of the members of the family Paramphistomidae only. G. discoides and Watsonius watsoni are responsible for the disease in humans, while most paramphistomes are responsible in livestock animals, and some wild mammals. In livestock industry the disease causes heavy economic backlashes due to poor production of milk, meat and wool.












